NovelMed Therapeutics is a clinical-stage biotechnology company focused on developing anti-complement therapies for rare (orphan) diseases.
|
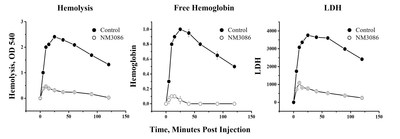
-- A potential for complete remission in PNH CLEVELAND, Dec. 12, 2022 /PRNewswire/ -- NovelMed Therapeutics, Inc. is a clinical-stage biotechnology company focused on developing anti-complement therapies for rare (orphan) diseases. NovelMed announced today that its lead anti-properdin antibody (NM3086) demonstrated top-line results in a rabbit model of Paroxysmal Nocturnal Hemoglobinuria (PNH). PNH is an orphan indication that is currently treated in part with C3 (Empaveli) and C5 (Soliris/Ultomiris) blockers. All three FDA-approved drugs block the entire complement system, resulting in undesirable effects such as persistent infections, anemia, and only partial remission from elevated lactate dehydrogenase (LDH) levels. Seeking Funds to Further Develop NM3086 as a Complete Treatment for PNH
NM3086 (an anti-properdin antibody) blocks the alternative pathway without blocking the classical pathway and as a result a) blocks hemolysis of PNH-like erythrocytes, b) reduces lactate dehydrogenase, and c) reduces free hemoglobin (Hb) in an animal model of PNH. Given the extent of this top-line data, Dr. Rekha Bansal, Chief Executive Officer, says "Efficacy of NM3086 in PNH patients is expected. "These positive results are promising and pave the way for evaluation of NM3086 as a potential monotherapy treatment and standard of care for PNH. NovelMed will continue to develop NM3086 in PNH while simultaneously exploring its immediate application in a range of other rare diseases where the complement system is dysregulated. PNH continues to be an unmet clinical need as none of the FDA-approved therapies have provided complete remission from existing complications." Results from the animal PNH model clearly underline the benefits and the importance of NM3086 as a treatment for PNH. NM3086's novel and well-differentiated mechanism of action is expected to result in a superior safety and efficacy profile compared to those FDA-approved for the treatment of PNH. "The animal data clearly highlight that NM3086 can control the mechanisms of both extravascular and intravascular hemolysis in PNH and may potentially change the way PNH is being treated," stated Robert Bard, Chief Regulatory Officer. NM3086 is a humanized monoclonal anti-properdin antibody that selectively blocks the alternative pathway (AP) without blocking the classical pathway (CP), which is critical for maintaining host defense against infections. AP dysregulation is the key mechanism responsible for the exacerbation of clinical symptoms occurring in PNH patients. These include a) excessive breakthrough hemolysis, b) increased levels of free hemoglobin, and c) increased levels of LDH. NM3086 is expected to control these clinical outcomes which continue to be a challenge for all PNH therapies currently approved by the US Food and Drug Administration. The AP also plays a key role in the initiation and propagation of inflammation and tissue damage that is manifested in several other complement-mediated diseases. NM3086 is being developed as a single drug that can potentially treat several rare and non-rare clinical conditions related to AP dysregulation. "Given the role of the AP in multiple complement-mediated rare diseases, NM3086 has the potential to treat several complement-mediated and complement-associated illnesses," states Richard Sutkus, the Intellectual Property Counsel. Seeking Funds to Further Develop NM3086 as a Robust Treatment for PNH PNH is a rare disease characterized by intravascular and extravascular hemolysis, higher LDH values, and lower hemoglobin values. In PNH patients currently treated with C5 blockers, approximately 36% of these patients still require more than one transfusion per year. In addition, almost all patients were found to be positive for opsonization markers while nearly 70% continued to have low hemoglobin levels despite treatment. NM3086 on the other hand is expected to fully control all of these AP related disease processes in PNH patients. NovelMed is currently seeking licensing, partnership, and acquisition opportunities to drive NM3086 through further development and approval in multiple rare disease indications. For more information, please visit www.NovelMed.com. Humanized Anti-Properdin Antibody (NM3086) NM3086 is a potent, first-in-class, humanized monoclonal antibody that is highly selective for properdin of the alternative pathway (AP) while sustaining the activity of the classical pathway (CP). Therefore, the drug label is not expected to have a Black Box warning that is associated with C3 and C5 blocking therapies. NM3086 is presently undergoing clinical development for several disorders related to anemia as well as for the treatment of PNH. In PNH, NM3086 acts upstream of the C3 and C5 terminal pathway, preventing not only intravascular hemolysis but also extravascular hemolysis via the alternative pathway (AP). In doing so, NM3086 aims for a therapeutic advantage over the current standard of care by targeting the underlying pathophysiology of PNH. NovelMed is moving NM3086 towards clinical development to evaluate this long acting anti-properdin antibody as a monotherapy for PNH patients. About NovelMed
NovelMed is innovating and developing medicines to improve and extend people's lives. As a clinical-stage biopharmaceutical company, we use innovative science to create transformative treatments in areas of rare disease. To discover these medicines, we consistently invest in the research and development of new generation anti-complement therapies. NovelMed has created a strong portfolio of intellectual property with broad applications to rare diseases. This includes the use of NM3086 as a treatment for a range of complement-mediated disorders related to uncontrolled activation of the AP. As part of its antibody platform, NM3086 will be evaluated in Phase II clinical trials in patients with multiple complement-mediated disorders. Our current anti-properdin portfolio includes bispecific anti-properdin antibodies with extended half-lives. NovelMed is currently seeking licensing, partnership, and acquisition opportunities to drive its antibody portfolio through further development and approval in multiple rare disease indications. For more information, please visit www.NovelMed.com. Contact:
SOURCE Novelmed Therapeutics |