Anika Therapeutics, Inc., a global joint preservation company focused on early intervention orthopedics, announced that Cingal® met its primary endpoint in a Phase III Study, demonstrating superiority over triamcinolone hexacetonide steroid alone at 26 weeks post-treatment.
- Study Demonstrated Superiority of Cingal, a Single-Injection Hyaluronic Acid-Based Viscosupplement Combined with Fast-Acting Steroid, Over Steroid Alone, for Osteoarthritis Pain Relief at 26 Weeks
- Study Builds on Prior Phase III Study Data that Demonstrated Superiority over Placebo and Hyaluronic Acid-Based Viscosupplement Alone
- Company Plans to Meet with FDA to Discuss Next Steps for U.S. Regulatory Approval
- Company Actively Assessing Options to Advance Cingal, Including Through Potential Commercial Partnerships
BEDFORD, Mass., Nov. 01, 2022 (GLOBE NEWSWIRE) -- Anika Therapeutics, Inc. (NASDAQ: ANIK), a global joint preservation company focused on early intervention orthopedics, today announced that Cingal® met its primary endpoint in a Phase III Study (Cingal 19-01), demonstrating superiority over triamcinolone hexacetonide (TH) steroid alone at 26 weeks post-treatment. The Cingal 19-01 Phase III study is the third completed Phase III study of Cingal and, together with previous studies, has now demonstrated superiority over each of its active ingredients and placebo. Across the three completed Phase III studies, Cingal demonstrated a mean 71% pain improvement from baseline and a mean 91% of Cingal subjects were deemed to be Responders.
Cingal is a combination product of cross-linked hyaluronic acid (HA) proven to provide long lasting pain relief through 6 months plus TH steroid to provide fast, short-term pain relief, both of which has been approved for sale in the United States, to treat the pain of knee osteoarthritis (OA). Cingal is currently sold in more than 35 countries outside the United States.
Anika will engage with the U.S. Food and Drug Administration (FDA) in the coming months on next steps for U.S. regulatory approval. In parallel, Anika is exploring the potential to advance Cingal through commercial partnerships in the U.S. and select Asian markets. These efforts will inform next steps, including if and how to proceed with another clinical trial in the United States.
“We are excited that Cingal successfully met the primary endpoint of this third Phase III study, and again confirmed the strong and durable pain relief observed in previous studies,” said Anika’s President and CEO, Cheryl R. Blanchard, Ph.D. “The successful results of this study represent a significant clinical milestone for Anika, and we look forward to meeting with the FDA to discuss next steps for U.S. regulatory approval. We believe that Cingal offers compelling clinical benefits to patients in terms of pain relief, durability of pain relief, responder rates and safety profile based on our established, real-world success with Cingal in over 35 countries outside the United States, as well as the totality of the data observed in our three completed Phase III studies. Cingal is a game-changing, next generation product for patients suffering from osteoarthritis, and we believe these clinical data position us well to further advance Cingal.”
Significant Market Opportunity Underscored by Successful Commercialization of Cingal in Over 35 Countries Outside the United States
Since 2016, Anika has successfully commercialized Cingal in over 35 countries outside the United States and controls full global rights. Anika is focused on further unlocking the clinical and commercial potential of Cingal in the United States and select Asian markets. A United States approval of Cingal by the FDA would significantly bolster Anika’s knee OA pain management portfolio with a market expanding, next generation OA product. Cingal has the potential to address a true unmet medical need for the more than 32 million patients in the United States suffering from osteoarthritis, with an estimated next generation OA U.S. market opportunity of at least $1 billion with additional expansion opportunities internationally.
Summary of the Cingal 19-01 Phase III Study Results
The Cingal 19-01 Phase III study was a multicenter, randomized, double-blind, parallel group, placebo-controlled, active comparator study that evaluated OA subjects at 26 sites in the United States. In total, 231 subjects were randomized into three treatment arms (Cingal (99 subjects), TH steroid alone (99 subjects) and placebo (saline) (33 subjects)). Anika conducted the Cingal 19-01 Phase III study with the aim of de-risking the Cingal clinical development program by demonstrating that a study that was placebo-controlled and enrolled patients with a higher pain score at baseline would show superiority of Cingal over TH steroid at 26 weeks.
The pre-specified primary analysis of the primary endpoint of the study was to evaluate change from baseline in knee pain as measured by the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Pain Index at 26 weeks post-treatment, comparing the Cingal arm to the TH steroid arm. WOMAC Pain Index is a widely used set of standardized questionnaires for health professionals to evaluate the condition of patients with osteoarthritis of the knee.
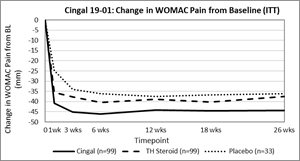
The 19-01 study met its primary endpoint and demonstrated superiority of Cingal over TH steroid at 26 weeks post-treatment based on change in WOMAC Pain Index from baseline (p=0.0406). The figure below shows the change in WOMAC Pain Index from baseline across all measured timepoints.
In addition, Cingal demonstrated strong performance for pain reduction with 66% improvement in WOMAC Pain Index (-44.3mm from baseline) and 90% of subjects were deemed to be OMERACT-OARSI Responders at 26 weeks post-treatment. OMERACT-OARSI is a recognized method of evaluating responder rates based on improvement in pain and function.
Cingal was well-tolerated in the study, with only transient and non-serious adverse events (e.g., arthralgia, injection site pain, swelling, stiffness) related to the study injections.
Summary of the Cingal Phase III Clinical Program
The Cingal 19-01 Phase III study is the third completed Phase III study of Cingal in OA patients. A total of 741 patients have been treated with Cingal in this clinical program yielding consistent and significant pain reduction for six months in this difficult to treat patient population.
In combination, Anika’s Phase III program has now demonstrated superiority of Cingal over each of its active ingredients and placebo. Cingal demonstrated superiority over the HA component (Monovisc®) at 1 week and 3 weeks post-treatment in the 13-01 study (p=0.0367 and p=0.0289), superiority over the TH steroid component at 26 weeks post-treatment in the 19-01 study (p=0.0406), and superiority over placebo at 12 weeks and 26 weeks post-treatment in the 13-01 study (p=0.0013 and p=0.0027).
Percent improvement for Cingal in change of WOMAC Pain Index from baseline at 26 weeks post-treatment was 72% for the 13-01 study, 73% for the 16-02 study, and 66% for the 19-01 study in a more difficult patient population (mean 71% improvement across all three studies). In addition, the percent of Cingal subjects who met criteria for the OMERACT-OARSI Responder Index at 26 weeks was 92% in the 13-01 study, 91% in the 16-02 study, and 90% in the 19-01 study (mean 91% Responders across all three studies). Cingal was well-tolerated with a consistent safety profile across all three studies, with no serious adverse events (SAEs) related to the Cingal injection.
About the FDA Requirements for Cingal NDA
The FDA previously advised Anika that it considers Cingal to be a drug-drug combination product that must meet the FDA’s fixed combination rule and thus demonstrate the contribution of each component to the therapeutic effect in a single, full factorial pivotal study. The agency has advised Anika that, because the TH steroid component is intended to provide pain reduction over the short-term while the HA component (Monovisc) is intended to be long-acting, it interprets this rule as requiring that Cingal demonstrate superior pain reduction over the two individual components at different time points (i.e., Cingal must demonstrate superiority on both the comparison to HA in the short term and the comparison to TH steroid in the long term) to support the intended treatment effect. As noted above, in combination, Anika’s Phase III program has now demonstrated superiority of Cingal over each of its active ingredients and placebo. Based on these data, Anika intends to engage with the FDA in the coming months on next steps for U.S. regulatory approval.
About Anika
Anika Therapeutics, Inc. (NASDAQ: ANIK), is a global joint preservation company that creates and delivers meaningful advancements in early intervention orthopedic care. Leveraging our core expertise in hyaluronic acid and implant solutions, we partner with clinicians to provide minimally invasive products that restore active living for people around the world. Our focus is on high opportunity spaces within orthopedics, including osteoarthritis pain management, regenerative solutions, sports medicine soft tissue repair and bone preserving joint technologies, and our products are efficiently delivered in key sites of care, including ambulatory surgery centers. Anika’s global operations are headquartered outside of Boston, Massachusetts. For more information about Anika, please visit www.anika.com.
ANIKA, ANIKA THERAPEUTICS, CINGAL, MONOVISC, and the Anika logo are registered trademarks of Anika Therapeutics, Inc. or its subsidiaries.
Forward-Looking Statements
This press release may contain forward-looking statements, within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, concerning the Company's expectations, anticipations, intentions, beliefs or strategies regarding the future which are not statements of historical fact, including statements about the results of the three completed Phase III clinical studies of Cingal (including Study 19-01), the clinical benefits and safety profile of Cingal, the timing and outcome of a planned meeting with the FDA about the U.S. regulatory pathway for Cingal, the U.S. regulatory pathway generally, including the need for additional Phase III pivotal studies, and, if approved, the commercialization of this product candidate. The FDA may require that the Company conduct a single, full factorial pivotal study of Cingal confirming the clinical results observed in the Phase III clinical trials completed to date prior to any regulatory submission for approval. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks, uncertainties, and other factors. The Company's actual results could differ materially from any anticipated future results, performance, or achievements described in the forward-looking statements as a result of a number of factors including, but not limited to, (i) the Company's ability to successfully commence and/or complete clinical trials of its products on a timely basis or at all; (ii) the Company's ability to obtain pre-clinical or clinical data to support domestic and international pre-market approval applications, 510(k) applications, or new drug applications, or to timely file and receive FDA or other regulatory approvals or clearances of its products; (iii) that such approvals will not be obtained in a timely manner or without the need for additional clinical trials, other testing or regulatory submissions, as applicable; (iv) the Company's research and product development efforts and their relative success, including whether we have any meaningful sales of any new products resulting from such efforts; (v) the cost effectiveness and efficiency of the Company's clinical studies, manufacturing operations, and production planning; (vi) the strength of the economies in which the Company operates or will be operating, as well as the political stability of any of those geographic areas; (vii) future determinations by the Company to allocate resources to products and in directions not presently contemplated; (viii) the Company's ability to successfully commercialize its products, in the U.S. and abroad; (ix) the Company's ability to provide an adequate and timely supply of its products to its customers; and (x) the Company's ability to achieve its growth targets. Additional factors and risks are described in the Company's periodic reports filed with the Securities and Exchange Commission, and they are available on the SEC's website at www.sec.gov. Forward-looking statements are made based on information available to the Company on the date of this press release, and the Company assumes no obligation to update the information contained in this press release.
For Investor Inquiries:
Mark Namaroff
Anika Therapeutics, Inc.
Vice President, Investor Relations, ESG and Corporate Communications
Direct: 781-457-9287
mnamaroff@anika.com
A chart accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e97cb1d2-9fd9-4f8c-96ad-e0a09fc059ee