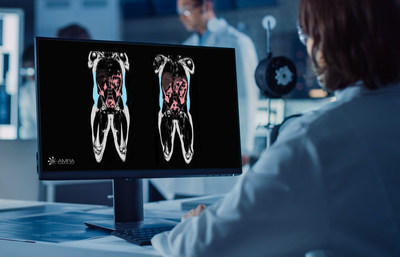
AMRA’s quantitative body composition method was used in Eli Lilly and Company’s magnetic resonance imaging (MRI) sub-study of the phase 3 SURPASS-3 clinical trial (trial NCT03882970) to assess the impact of tirzepatide (5 mg, 10 mg, 15 mg) on various fat depots in individuals with Type 2 Diabetes (T2DM).
|
Eli Lilly and Company assessed the impact of tirzepatide, an investigational therapy for the treatment of type 2 diabetes, on liver fat, visceral fat, and subcutaneous adipose tissue in an MRI sub-study of the phase 3 SURPASS-3 clinical trial LINKÖPING, Sweden, April 26, 2022 /PRNewswire/ -- AMRA's quantitative body composition method was used in Eli Lilly and Company's magnetic resonance imaging (MRI) sub-study of the phase 3 SURPASS-3 clinical trial (trial NCT03882970) to assess the impact of tirzepatide (5 mg, 10 mg, 15 mg) on various fat depots in individuals with Type 2 Diabetes (T2DM). The results were recently published in The Lancet Diabetes & Endocrinology. SURPASS-3 was a 52-week, multi-center, randomized, phase 3, open-label trial. The SURPASS-3 MRI sub-study compared the effect of tirzepatide versus titrated insulin degludec on liver fat content, the volume of visceral fat, and the volume of abdominal subcutaneous adipose tissue in 296 individuals with T2DM. Lilly included AMRA's MRI-based body composition methods in the sub-study to help characterize and understand the potential changes in visceral and subcutaneous fat content in response to treatment. At 52 weeks, treatment with tirzepatide lowered liver fat with a concurrent reduction of visceral fat and subcutaneous fat. With insulin degludec, liver fat was also reduced (to a lesser extent compared to tirzepatide), however, this occurred with an increase in visceral and subcutaneous fat. Recent research has shown the relevance of assessing different fat depots due to the different cardiovascular risk associated with the balance between them. Eric Converse, CEO at AMRA, commented: "We are on a mission to support companies in assessing more holistic effects of their investigational therapies. Our MRI-based services allowed Lilly to assess volume of visceral fat and abdominal subcutaneous adipose tissue. The results of Lilly's sub-study enabled further characterization of the metabolic effect of tirzepatide beyond the commonly measured changes in weight and BMI." Further analyses will evaluate if specific areas of adipose tissue are being targeted by tirzepatide and assess the changes in body composition and cardiometabolic profiles. Pharmaceutical companies can get additional insights from AMRA's MRI methods in late-stage clinical trials. Learn how AMRA can help you advance the development of therapeutics for metabolic and musculoskeletal diseases by visiting AMRA’s website or connecting with our team.
SOURCE AMRA Medical |