WARRINGTON, Pa., Oct. 24, 2016 /PRNewswire/ -- Windtree Therapeutics, Inc. (Nasdaq: WINT), a biotechnology company focused on developing aerosolized KL4 surfactant therapies for respiratory diseases, today released data from a lung deposition study conducted in non-human primates (NHPs) that demonstrates the Company's proprietary aerosol delivery system (ADS) is capable of delivering aerosolized KL4 surfactant throughout all regions of the lung. The study consisted of a series of experiments in NHPs designed to assess the distribution and deposition of aerosolized KL4 surfactant in the lung when administered using the ADS. The Company is developing the ADS as part of its lead development program, AEROSURF® (lucinactant for inhalation). AEROSURF is currently being studied in a phase 2b clinical trial as a potential noninvasive treatment for respiratory distress syndrome (RDS) in premature infants. The Company will host a conference call and live webcast on Tuesday, October 25, 2016 at 8:00 a.m. EDT to provide additional details of the study results as well as provide an update on the AEROSURF phase 2 program.
"These study results confirm the ability of our aerosol delivery technology to overcome the barriers of successfully aerosolizing and delivering a surfactant with particle sizes appropriate for deep lung delivery with uniform distribution across all regions of the lung," commented Craig Fraser, President and Chief Executive Officer. "These results not only support the premise of our AEROSURF RDS program, but also complement the clinical evidence seen in our phase 2a trial in premature infants 29 to 34 week gestational age and provide further insight into other potential applications of this novel technology in the future."
Data from this study were generated from an in vivo distribution study using three NHPs, cynomolgus macaques, which received three-to-ten minute exposures of technetium-labeled KL4 surfactant that was aerosolized using the same model ADS currently being used in the AEROSURF phase 2b clinical program. After administration, researchers immediately acquired 2-D planar images followed by 3-D SPECT images and then a second 2-D planar image to assess overall pulmonary distribution. Additionally, the 3-D SPECT lung data were analyzed using a quantitative methodology whereby regional distribution was assessed across ten equally sized shells (or layers) of the lung, from the innermost shell through the outermost shell.
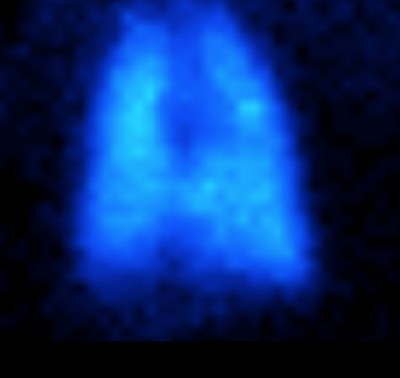
Results from the analysis of the 2-D planar and 3-D SPECT images show that aerosolized KL4 surfactant, delivered using the ADS via constant flow nasal continuous positive airway pressure (nCPAP), was generally uniformly deposited in all regions of the NHPs lungs (Image 1 right).
Results from a quantitative analysis further demonstrated that there was generally uniform distribution in all regions of the lung, with an average total lung distribution of 52 percent in the five inner shells and 48 percent in the five outer shells (Chart 1 right).
"We are extremely pleased with the results of this study because, along with other work we have done, it serves as yet another validation of the potentially transformational capabilities of our ADS device, which aerosolizes our KL4 surfactant in a consistent and controlled flow and delivers it throughout the lungs to the areas where a surfactant needs to reach to produce its beneficial effects," commented Steve Simonson, M.D, Senior Vice President and Chief Development Officer.
Conference Call and Webcast Details
The Company will host a conference call and webcast (including a slide presentation) at 8:00 a.m. EDT on Tuesday, October 25, 2016 to provide updates on the AEROSURF® phase 2 clinical program and the lung deposition study in non-human primates.
To participate in the live call and take part in the question and answer session, dial (844) 802-2436 (domestic) or (412) 317-5129 (international). The live webcast, including a slide presentation, can be accessed at http://windtreetx.investorroom.com/events.
A replay of the conference call will be accessible one hour after completion through November 1, 2016 by dialing (877) 344-7529 (domestic) or (412) 317-0088 (international) and referencing conference number 10095419. An archive of the webcast can be accessed on the Company's website at http://windtreetx.investorroom.com/events.
About AEROSURF®
Windtree's lead product candidate is AEROSURF (lucinactant for inhalation), a novel, investigational combination drug/device product that combines the Company's proprietary KL4 surfactant and aerosolization technologies. AEROSURF is being developed to potentially reduce or eliminate the need for endotracheal intubation and mechanical ventilation in the treatment of premature infants with respiratory distress syndrome (RDS). Enrollment is ongoing in a phase 2b clinical trial to study AEROSURF administered to premature infants receiving nasal continuous positive airway pressure (nCPAP) for RDS, compared to infants receiving nCPAP alone. The phase 2b trial is a global trial with clinical sites in North America, Europe and Latin America.
About Windtree Therapeutics
Windtree Therapeutics, Inc. is a clinical-stage biotechnology company focused on developing novel surfactant therapies for respiratory diseases and other potential applications. Windtree's proprietary technology platform includes a synthetic, peptide-containing surfactant (KL4 surfactant) that is structurally similar to endogenous pulmonary surfactant and novel drug-delivery technologies being developed to enable noninvasive administration of aerosolized KL4 surfactant. Windtree is focused initially on improving the management of respiratory distress syndrome (RDS) in premature infants and believes that its proprietary technology may make it possible, over time, to develop a pipeline of KL4 surfactant product candidates to address a variety of respiratory diseases for which there are few or no approved therapies.
For more information, please visit the Company's website at www.windtreetx.com.
Forward-Looking Statements
To the extent that statements in this press release are not strictly historical, all such statements are forward-looking, and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from the statements made. Examples of such risks and uncertainties include those risks related to Windtree's aerosolized KL4 surfactant development programs, including for AEROSURF, which may involve time-consuming and expensive clinical trials that may be subject to potentially significant delays or regulatory holds, or fail; risks related to the development of aerosol delivery systems (ADS) and related components; risks related to the manufacture by contract manufacturers or suppliers of drug products, drug substances, ADS and other materials on a timely basis and in sufficient amounts; risks relating to rigorous regulatory requirements, including those of the U.S. Food and Drug Administration or other regulatory authorities that may require significant additional activities, or may not accept or may withhold or delay consideration of applications, or may not approve or may limit approval of Windtree's products; and other risks and uncertainties described in Windtree's filings with the Securities and Exchange Commission including the most recent reports on Forms 10-K, 10-Q and 8-K, and any amendments thereto.
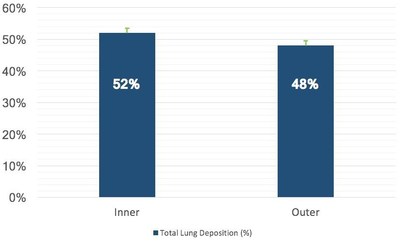
Photo - http://photos.prnewswire.com/prnh/20161024/431836
Photo - http://photos.prnewswire.com/prnh/20161024/431837
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/windtree-releases-data-from-lung-deposition-study-in-non-human-primates-demonstrating-uniform-distribution-of-aerosolized-kl4-surfactant-in-all-regions-of-the-lung-300349990.html
SOURCE Windtree Therapeutics, Inc.





