Vanda Pharmaceuticals Inc. reported that interim analysis showed tradipitant may accelerate clinical improvement in SARS-CoV-2 pneumonia in the ODYSSEY study.
WASHINGTON, Aug. 18, 2020 /PRNewswire/ -- Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) today reported that interim analysis showed tradipitant may accelerate clinical improvement in SARS-CoV-2 (COVID-19) pneumonia in the ODYSSEY study.
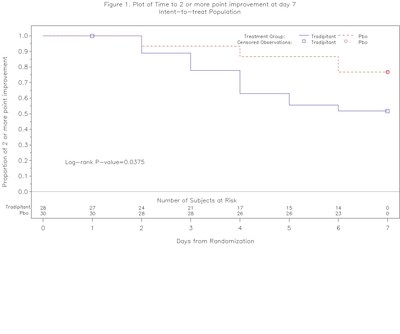
- Interim analysis of the ODYSSEY study showed that a 14 day tradipitant treatment accelerated clinical improvement by day 7 (HR=2.55, p=0.0375)
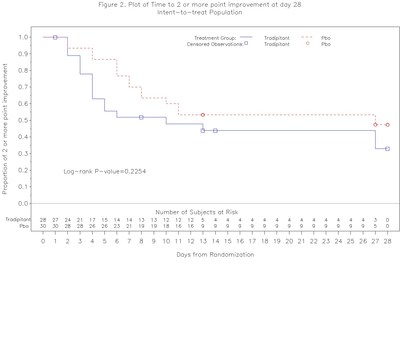
- Tradipitant numerically improved median time to clinical improvement by day 28 (HR=1.55, p=0.2254)
- Similar overall rates of improvement and mortality were observed for the two treatment arms at day 28
The interim analysis of the ODYSSEY study demonstrated that hospitalized patients with COVID-19 pneumonia improved sooner when treated with tradipitant as compared to placebo. This finding was based on a preliminary analysis of the first 60 patients enrolled in the ODYSSEY study of tradipitant in COVID-19 pneumonia. ODYSSEY is an ongoing Phase III double-blind placebo-controlled trial investigating the efficacy and safety of tradipitant, a neurokinin-1 receptor (NK-1R) antagonist, in the treatment of neurogenic inflammation of the lung secondary to SARS-CoV-2 (COVID-19) infection, which was initiated in April 2020. The study is expected to enroll 300 patients, and as of July 15, 2020, 60 patients had enrolled and completed the study. Because this is the first study of tradipitant for this indication and given the increased rate of mortality seen with COVID-19 pneumonia, an interim analysis was planned to better assess the efficacy and safety of tradipitant in this population of COVID-19 patients.
In the ODYSSEY study, clinical status was assessed on a 7 point scale ranging from death, to mechanical ventilation, various levels of oxygen requirements, to hospital discharge. Clinical improvement was defined as at least a 2 point improvement in the 7 point ordinal scale.
- Interim analysis in the first 60 enrolled patients showed that similar percentages of patients improved between the two treatment arms, 57% for tradipitant and 50% for placebo. The mortality rate was also similar between the treatment groups with 14.2% for tradipitant and 16.6% for placebo.
- In the time to improvement analysis, after 7 days of treatment, patients treated with tradipitant recovered earlier than those receiving placebo, which was statistically significant (HR=2.55, p=0.0375). This benefit was generally consistent among patients of varying degree of severity at baseline. At day 28 of the study, tradipitant showed a numerical benefit over placebo with an earlier median time to recovery (HR=1.55, p=0.2254, median time to improvement 10 days for tradipitant and 28 days for placebo).
This early analysis suggests that tradipitant may act by accelerating the time to clinical improvement for patients with severe COVID-19 pneumonia. If confirmed, this effect may be of significant clinical benefit for patients as well as for public health by decreasing the amount of resources employed in the treatment of patients with COVID-19 pneumonia. Although preliminary, the interim results from this randomized controlled study of tradipitant in COVID-19 pneumonia are encouraging. A larger sample size would be required to definitively determine whether tradipitant offers a therapeutic benefit in hospitalized patients with COVID-19 pneumonia by accelerating time to clinical improvement. The results from this interim analysis will be submitted for publication in a peer reviewed journal.
The hypothesized mechanism of action of tradipitant as an anti-inflammatory agent in COVID-19 pneumonia potentially would be complementary to antiviral treatments. Ongoing efforts in the development of COVID-19 therapeutics require coordination and cooperation between parties if they are to result in the discovery of useful therapeutics. Vanda looks forward to collaborating with U.S. government agencies and hospitals across the country to confirm these findings expediently. If these results are confirmed, tradipitant could become part of the standard of care, either alone or in combination with antivirals, for patients with COVID-19 pneumonia.
"These results, albeit preliminary, are exciting, offering the promise of a significant contribution in the treatment of COVID-19 and the prospect of making tradipitant part of the standard of care in accelerating recovery for patients with COVID-19 pneumonia," said Mihael H. Polymeropoulos, M.D., President and CEO of Vanda.
Vanda has scaled up the commercial manufacturing of tradipitant and significant supplies are expected to be available in the coming months. As the results today may suggest that tradipitant's effects in accelerating recovery may not be restricted to just COVID-19 pneumonia, Vanda also plans to evaluate a clinical program to assess the efficacy of tradipitant in the treatment of seasonal influenza pneumonia.
|
Table 1. Baseline Demographic Summary. Intention-to-Treat Population |
|||||
|
Characteristic |
Tradipitant |
Placebo |
Total |
||
|
Statistic |
(N= 28) |
(N= 30) |
(N= 58) |
||
|
Age (years) |
71.0 (62.5 - 77.5) |
66.5 (58.0 - 72.0) |
68.5 (61.0 - 77.0) |
||
|
Sex, n (%) |
|||||
|
Male |
20 ( 71.4) |
20 ( 66.7) |
40 ( 69.0) |
||
|
Female |
8 ( 28.6) |
10 ( 33.3) |
18 ( 31.0) |
||
|
Any Comorbidities, n (%) |
27 ( 96.4) |
28 ( 93.3) |
55 ( 94.8) |
||
|
Hypertension |
11 ( 39.3) |
15 ( 50.0) |
26 ( 44.8) |
||
|
Diabetes |
8 ( 28.6) |
7 ( 23.3) |
15 ( 25.9) |
||
|
Coronary Heart Disease |
1 ( 3.6) |
4 ( 13.3) |
5 ( 8.6) |
||
|
Asthma |
1 ( 3.6) |
4 ( 13.3) |
5 ( 8.6) |
||
|
7 Point Ordinal Scale at Baseline, n (%) |
|||||
|
2 - Hospitalized on mechanical ventilation or ECMO |
4 ( 14.3) |
2 ( 6.7) |
6 ( 10.3) |
||
|
3 - Hospitalized on non-invasive ventilation or high-flow oxygen supplement |
13 ( 46.4) |
12 ( 40.0) |
25 ( 43.1) |
||
|
4 - Hospitalized requiring supplemental oxygen |
9 ( 32.1) |
16 ( 53.3) |
25 ( 43.1) |
||
|
5 - Hospitalized not requiring supplemental oxygen, requiring continued medical care |
2 ( 7.1) |
0 ( 0.0) |
2 ( 3.4) |
||
|
Time from Hospitalization to Starting Study Treatment, Days |
4.0 (2.0 - 6.5) |
6.0 (2.0 - 12.0) |
4.0 (2.0 - 9.0) |
||
|
Early (<=10 Days from Hospitalization) |
23 ( 82.1) |
22 ( 73.3) |
45 ( 77.6) |
||
|
Late (>10 Days from Hospitalization) |
5 ( 17.9) |
8 ( 26.7) |
13 ( 22.4) |
||
|
Highest Oxygen Therapy Support , n (%) |
|||||
|
Room Air |
1 ( 3.6) |
0 ( 0.0) |
1 ( 1.7) |
||
|
Nasal Cannula (NC) |
5 ( 17.9) |
6 ( 20.0) |
11 ( 19.0) |
||
|
Non Rebreather (NRB) |
1 ( 3.6) |
2 ( 6.7) |
3 ( 5.2) |
||
|
High Flow Nasal Cannula (HFNC) |
6 ( 21.4) |
9 ( 30.0) |
15 ( 25.9) |
||
|
CPAP Mask |
0 ( 0.0) |
2 ( 6.7) |
2 ( 3.4) |
||
|
BiPAP Mask |
1 ( 3.6) |
1 ( 3.3) |
2 ( 3.4) |
||
|
Mechanical Ventilation |
14 ( 50.0) |
10 ( 33.3) |
24 ( 41.4) |
||
|
% = 100 x n/N. Data are median (IQR). |
|||||
|
Table 2. Outcomes Overall and According to Score on the Ordinal Scale at Day 7. Intention-to-Treat Population |
||||||||||||||||
|
Overall* |
Ordinal Score at Baseline* |
|||||||||||||||
|
4 |
3 |
2 |
||||||||||||||
|
Tradipitant |
Placebo |
Tradipitant |
Placebo |
Tradipitant |
Placebo |
Tradipitant |
Placebo |
|||||||||
|
(N= 28) |
(N= 30) |
(N= 9) |
(N= 16) |
(N= 13) |
(N= 12) |
(N= 4) |
(N= 2) |
|||||||||
|
Responder as Improvement of 2 or More Points |
||||||||||||||||
|
No. of responders |
13 |
7 |
6 |
6 |
2 |
1 |
3 |
0 |
||||||||
|
Median time to responder |
. (4-NE) |
. (NE-NE) |
4 (3-NE) |
. (4-NE) |
. (5-NE) |
. (NE-NE) |
4 (2-NE) |
. (NE-NE) |
||||||||
|
(95% CI) — days |
||||||||||||||||
|
Hazard ratio (95% CI)** |
2.55 (1.02-6.42 [0.0461]) |
2.23 (0.71-6.98 [0.1673]) |
2.19 (0.20-24.18 [0.5225]) |
NE (0.00-NE [0.9983]) |
||||||||||||
|
Mortality |
||||||||||||||||
|
Hazard ratio (95% CI)† |
2.65 (0.24-29.29 [0.4255]) |
3.14 (0.20-50.23 [0.4186]) |
. (NE - NE [NE]) |
NE (0.00-NE [0.9985]) |
||||||||||||
|
No. of deaths by day 7 |
2 |
1 |
1 |
1 |
0 |
0 |
1 |
0 |
||||||||
|
KM estimate — % |
9.8 |
3.8 |
25.0 |
8.3 |
0.0 |
0.0 |
25.0 |
0.0 |
||||||||
|
(95% CI) |
(2.5-33.8) |
(0.6-24.3) |
(3.9-87.2) |
(1.2-46.1) |
(0.0-0.0) |
(0.0-0.0) |
(3.9-87.2) |
(0.0-0.0) |
||||||||
|
Ordinal Score at Day 7 Days — no. (%)‡ |
||||||||||||||||
|
Patients with baseline |
28 |
30 |
9 |
16 |
13 |
12 |
4 |
2 |
||||||||
|
and day 7 score data |
||||||||||||||||
|
1 |
2 (7.1) |
1 (3.3) |
1 (11.1) |
1 (6.3) |
0 (0.0) |
0 (0.0) |
1 (25.0) |
0 (0.0) |
||||||||
|
2 |
9 (32.1) |
8 (26.7) |
0 (0.0) |
2 (12.5) |
8 (61.5) |
4 (33.3) |
1 (25.0) |
2 (100.0) |
||||||||
|
3 |
4 (14.3) |
5 (16.7) |
1 (11.1) |
1 (6.3) |
3 (23.1) |
4 (33.3) |
0 (0.0) |
0 (0.0) |
||||||||
|
4 |
2 (7.1) |
9 (30.0) |
1 (11.1) |
6 (37.5) |
0 (0.0) |
3 (25.0) |
1 (25.0) |
0 (0.0) |
||||||||
|
5 |
2 (7.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (7.7) |
0 (0.0) |
1 (25.0) |
0 (0.0) |
||||||||
|
7 |
9 (32.1) |
7 (23.3) |
6 (66.7) |
6 (37.5) |
1 (7.7) |
1 (8.3) |
0 (0.0) |
0 (0.0) |
||||||||
|
Odds ratio (95% CI) |
1.05 (0.42-2.63 [0.9149]) |
0.40 (0.08-2.02 [0.2692]) |
2.69 (0.60-12.18 [0.1980]) |
0.43 (0.02-11.53 [0.6180]) |
||||||||||||
|
* |
P values and confidence intervals have not been adjusted for multiple comparisons. NE denotes not possible to estimate. |
|
** |
Hazard ratios was calculated from the Cox model; P values for hazard ratios were calculated with the log-rank test. Hazard ratios bigger than 1 indicate a benefit for tradipitant. |
|
† |
Hazard ratios was calculated from the Cox model; P values for hazard ratios were calculated with the log-rank test. Hazard ratios less than 1 indicate a benefit for tradipitant. |
|
‡ |
Odds ratios and P values were calculated with the use of a proportional odds model. Odds ratio values greater than 1 indicate a benefit for tradipitant. |
|
Table 3. Outcomes Overall and According to Score on the Ordinal Scale at Day 28. Intention-to-Treat Population |
|||||||||||||||
|
Overall* |
Ordinal Score at Baseline* |
||||||||||||||
|
4 |
3 |
2 |
|||||||||||||
|
Tradipitant |
Placebo |
Tradipitant |
Placebo |
Tradipitant |
Placebo |
Tradipitant |
Placebo |
||||||||
|
(N= 28) |
(N= 30) |
(N= 9) |
(N= 16) |
(N= 13) |
(N= 12) |
(N= 4) |
(N= 2) |
||||||||
|
Responder as Improvement of 2 or More Points |
|||||||||||||||
|
No. of responders |
16 |
15 |
7 |
11 |
4 |
4 |
3 |
0 |
|||||||
|
Median time to responder |
10 (4-NE) |
27 (8-NE) |
4 (3-NE) |
9 (4-NE) |
27 (5-NE) |
. (7-NE) |
4 (2-NE) |
. (NE-NE) |
|||||||
|
(95% CI) — days |
|||||||||||||||
|
Hazard ratio (95% CI)** |
1.55 (0.76-3.14 [0.2267]) |
1.59 (0.61-4.15 [0.3396]) |
1.08 (0.27-4.35 [0.9086]) |
NE (0.00- [0.9983]) |
|||||||||||
|
Mortality |
|||||||||||||||
|
Hazard ratio (95% CI)† |
1.03 (0.28-3.85 [0.9610]) |
1.24 (0.13-11.99 [0.8543]) |
0.95 (0.06-15.26 [0.9731]) |
1.84 (0.15-22.52 [0.6328]) |
|||||||||||
|
No. of deaths by day 28 |
4 |
5 |
1 |
3 |
1 |
1 |
2 |
1 |
|||||||
|
KM estimate — % |
20.4 |
25.9 |
25.0 |
35.8 |
9.1 |
11.1 |
50.0 |
50.0 |
|||||||
|
(95% CI) |
(8.2-45.7) |
(11.5-52.2) |
(3.9-87.2) |
(12.4-77.5) |
(1.3-49.2) |
(1.6-56.7) |
(15.5-94.2) |
(9.0-99.4) |
|||||||
|
Ordinal Score at Day 28 Days — no. (%)‡ |
|||||||||||||||
|
Patients with baseline |
28 |
30 |
9 |
16 |
13 |
12 |
4 |
2 |
|||||||
|
and day 28 score data |
|||||||||||||||
|
1 |
4 (14.3) |
5 (16.7) |
1 (11.1) |
3 (18.8) |
1 (7.7) |
1 (8.3) |
2 (50.0) |
1 (50.0) |
|||||||
|
2 |
8 (28.6) |
5 (16.7) |
0 (0.0) |
1 (6.3) |
8 (61.5) |
3 (25.0) |
0 (0.0) |
1 (50.0) |
|||||||
|
3 |
1 (3.6) |
2 (6.7) |
1 (11.1) |
0 (0.0) |
0 (0.0) |
2 (16.7) |
0 (0.0) |
0 (0.0) |
|||||||
|
4 |
0 (0.0) |
3 (10.0) |
0 (0.0) |
1 (6.3) |
0 (0.0) |
2 (16.7) |
0 (0.0) |
0 (0.0) |
|||||||
|
7 |
15 (53.6) |
15 (50.0) |
7 (77.8) |
11 (68.8) |
4 (30.8) |
4 (33.3) |
2 (50.0) |
0 (0.0) |
|||||||
|
Odds ratio (95% CI) |
1.01 (0.38-2.67 [0.9795]) |
0.62 (0.09-4.02 [0.6119]) |
2.21 (0.50-9.71 [0.2941]) |
0.43 (0.02-12.22 [0.6242]) |
|||||||||||
|
* |
P values and confidence intervals have not been adjusted for multiple comparisons. NE denotes not possible to estimate. |
|
** |
Hazard ratios was calculated from the Cox model; P values for hazard ratios were calculated with the log-rank test. Hazard ratios bigger than 1 indicate a benefit for tradipitant. |
|
† |
Hazard ratios was calculated from the Cox model; P values for hazard ratios were calculated with the log-rank test. Hazard ratios less than 1 indicate a benefit for tradipitant. |
|
‡ |
Odds ratios and P values were calculated with the use of a proportional odds model. Odds ratio values greater than 1 indicate a benefit for tradipitant. |
About Tradipitant
Tradipitant is an NK-1R antagonist licensed by Vanda from Eli Lilly and Company. Tradipitant is currently in clinical development for gastroparesis, motion sickness and atopic dermatitis. The FDA has imposed a partial clinical hold on tradipitant clinical protocols of longer than 12 weeks duration.
About Vanda Pharmaceuticals Inc.
Vanda is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. For more on Vanda Pharmaceuticals Inc., please visit www.vandapharma.com and follow us on Twitter @vandapharma.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Various statements in this press release, including, but not limited to statements regarding the potential for tradipitant to be a safe and effective treatment for COVID-19 pneumonia and seasonal influenza pneumonia and Vanda's ability to make tradipitant available to patients for the treatment of COVID-19 pneumonia and seasonal influenza pneumonia are "forward-looking statements" under the securities laws. Forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. Important factors that could cause actual results to differ materially from those reflected in Vanda's forward-looking statements include, among others, Vanda's ability to fully enroll and complete the ODYSSEY study and Vanda's ability to complete the development of, and obtain regulatory approval for, tradipitant for the treatment of COVID-19 pneumonia and seasonal influenza pneumonia. There can be no assurance that the actual results or developments anticipated by Vanda will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Vanda. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved. Forward-looking statements in this press release should be evaluated together with the various risks and uncertainties that affect Vanda's business and market, particularly those identified in the "Cautionary Note Regarding Forward-Looking Statements", "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Vanda's Annual Report on Form 10-K for the fiscal year ended December 31, 2019, as updated by Vanda's subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the U.S. Securities and Exchange Commission, which are available at www.sec.gov.
All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this press release is provided only as of the date of this press release, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Corporate Contact:
AJ Jones II
Chief Corporate Affairs and Communications Officer
Vanda Pharmaceuticals Inc.
202-734-3400
pr@vandapharma.com
Elizabeth Van Every
Head of Corporate Affairs
Vanda Pharmaceuticals Inc.
202-734-3400
pr@vandapharma.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/vanda-pharmaceuticals-interim-analysis-from-odyssey-study-shows-tradipitant-may-accelerate-clinical-improvement-in-patients-with-covid-19-pneumonia-301113676.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/vanda-pharmaceuticals-interim-analysis-from-odyssey-study-shows-tradipitant-may-accelerate-clinical-improvement-in-patients-with-covid-19-pneumonia-301113676.html
SOURCE Vanda Pharmaceuticals Inc.
Company Codes: NASDAQ-NMS:VNDA