Uro Medical Corporation announced the acquisition of Micron Medical Corporation’s urology-based assets, including the continuation of the Protect randomized controlled trial, which is a head to head study comparing tibial stimulation to sacral stimulation.
Uro Medical’s breakthrough injectable neuromodulation product, Protect PNS, is currently under regulatory review by the FDA for market approval
BOCA RATON, Fla., July 06, 2021 (GLOBE NEWSWIRE) -- Uro Medical Corporation, a privately-held urology-focused medical device company engaged in the development, manufacture, and commercialization of innovative, minimally invasive neurostimulation solutions, today announced the acquisition of Micron Medical Corporation’s urology-based assets, including the continuation of the Protect randomized controlled trial (RCT), which is a head to head study comparing tibial stimulation to sacral stimulation.
“Our new organization – Uro Medical Corporation – reflects our core business focus in urology and improving how neuromodulation is delivered to treat previously under-served urologic conditions," said Matt Kemp, Chief Commercial Officer of Uro Medical. "As a company with its foundation in neuromodulation technology, we are committed to delivering breakthrough urology products, like our Protect PNS system, that we believe will increase access, lower costs and improve outcomes for millions of patients forced to seek bladder protection solutions. With our Protect PNS market approval application currently under review by the U.S. Food and Drug Administration (FDA) for the treatment of patients with refractory overactive bladder (OAB), we believe we are well-positioned to deliver on the company’s urology-focused mission.”
In addition, Uro Medical announced the initiation of the Guardian clinical trial, a multi-center randomized controlled clinical trial evaluating the safety and efficacy of Protect PNS as compared to traditional medical management for the treatment for OAB. The Guardian study, which is evaluating the efficacy of tibial stimulation compared to pharmaceutical medical management, is expected to enroll approximately 600 subjects with refractory OAB across multiple clinical sites in the United States. The results of this study, which is the second randomized clinical trial conducted for the Protect PNS device, will be used to support long term, nationwide payor reimbursement coverage as a mainline therapy option.
“We are excited to partner with Uro Medical as the first clinical trial site in the country to enroll for this pivotal clinical trial supporting the overall Protect PNS program,” said Dr. Larry Sirls, from Comprehensive Urology in Royal Oak, MI. “Based on the data generated to date in the earlier trials and it’s compelling product profile as an injectable device, Protect PNS has the potential to revolutionize how OAB patients are treated and become the standard of care.”
Mr. Kemp continued, “With our evolution into a fully-focused urology company complete, we are firmly dedicated to securing FDA approval of our pioneering wireless, minimally invasive, microtechnology neurostimulator, Protect PNS, for the treatment of OAB, a condition that affects over 37 million Americans, and preparing for its U.S. commercial launch.”
Learn more about Uro Medical Corporation at www.uromedical.com.
About Protect PNS
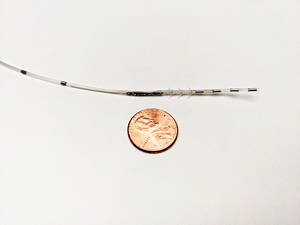
Protect PNS is a wirelessly powered, minimally invasive, microtechnology neurostimulator intended to treat overactive bladder (OAB). Protect PNS is currently being studied for the treatment of OAB and is under regulatory review for market approval by the FDA.
About Overactive Bladder
OAB is a clinical condition that occurs when the bladder muscle contracts involuntarily. Symptoms may include urinary urgency (the sudden urge to urinate that is difficult to control), urgency incontinence (unintentional loss of urine immediately after an urgent need to urinate), frequent urination (usually eight or more times in 24 hours), and nocturia (waking up more than two times in the night to urinate).1Approximately 37 million Americans suffer from symptoms of OAB, which can have a significant impairment on a patient’s day-to-day activities.1,2
About Uro Medical
Uro Medical is a privately held medical device company engaged in the development, manufacture, and commercialization of wirelessly powered, microtechnology neurostimulators, providing patients with convenient, safe, minimally invasive, and highly cost-effective urological solutions that are easily incorporated into their daily lives. Uro Medical’s goal is to evolve its patented, cutting-edge platform for neuromodulation to standard of care, increasing the accessibility for patients worldwide while lowering the economic impact of urology care management. www.uromedical.com
- Reynolds, W. S., Fowke, J., & Dmochowski, R. (2016). The Burden of Overactive Bladder on US Public Health. Current bladder dysfunction reports, 11(1), 8–13. https://doi.org/10.1007/s11884-016-0344-9
- Coyne, K. S., Sexton, C. C., Vats, V., Thompson, C., Kopp, Z. S., & Milsom, I. (2011). National community prevalence of overactive bladder in the United States stratified by sex and age. Urology, 77(5), 1081–1087. https://doi.org/10.1016/j.urology.2010.08.039
| Contacts | |
| Gil Bao | Jeremy Feffer |
| Uro Medical | LifeSci Advisors |
| info@uromedical.com | jeremy@lifesciadvisors.com |
| 888.691.0585 | (212) 915-2568 |
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/394d446b-a0f3-414a-a105-ae76e9e16413