Upstream Peripheral Technologies announced that its CTO-busting GoBack® Catheter for crossing and reentry was proven very effective for patients undergoing complex revascularizations in lower limb arteries.
100-patient study confirms efficiency of Upstream Peripheral’s GoBack Catheter to cross toughest peripheral vascular lesions
CAESAREA, Israel & TAMPA, Fla.--(BUSINESS WIRE)-- Upstream Peripheral Technologies announced today that its CTO-busting GoBack® Catheter for crossing and reentry was proven very effective for patients undergoing complex revascularizations in lower limb arteries. The findings are based on a peer-reviewed study published in the Journal of Endovascular Therapy.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220104005157/en/
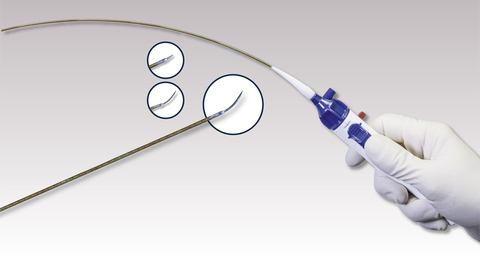
The GoBack Catheter is a single-lumen crossing catheter which features a curved nitinol needle that serves as an effective crossing tool. The versatile needle can be extended straight or to a curved position beyond the GoBack Catheter’s tip. The protrusion length is determined by the clinician with a thumb selector on the device’s handle. The GoBack comes in two configurations of 4 French and 2.9 French for above and below the knee procedures. The GoBack has regulatory approval in 30 countries. (Photo: Upstream Peripheral Technologies)
In a single-center retrospective study led by Professor Andrej Schmidt (University Hospital, Leipzig), researchers reviewed outcomes of 100 consecutive patients who underwent treatment with the GoBack Catheter after failed crossing attempts using standard guidewire and support catheter techniques. All lesions were confirmed as either de-novo or re-occluded chronic total occlusions (CTO). The overall technical success rate was 92%.
The study highlights the versatility and effectiveness of the GoBack Catheter as both a crossing and reentry tool when compared to alternative products. The study further outlines the clinical benefits of the GoBack Catheter with its robust, retractable needle at its tip.
Dani Rottenberg, founder and CEO of Upstream, remarked, "All patients should have access to the most effective endovascular revascularization technologies to avoid unnecessary bypass surgeries and amputations. The GoBack helps clinicians achieve such a goal, especially in more complex lesions.”
With these positive results, Upstream plans to make the GoBack available to hospitals and peripheral vascular catheterization labs throughout the world. Rottenberg explains, “The GoBack helps facilities manage their costs and maximize their lab optimization by reducing procedure time and using less consumables per procedure.”
The GoBack Catheter is a single-lumen crossing catheter which features a curved nitinol needle that serves as an effective crossing tool. The versatile needle can be extended straight or to a curved position beyond the GoBack Catheter’s tip. The protrusion length is determined by the clinician with a thumb selector on the device’s handle. The GoBack comes in two configurations of 4 French and 2.9 French for above and below the knee procedures. The GoBack has regulatory approval in 30 countries.
About the company:
Upstream Peripheral Technologies, a privately-held company, produces the GoBack Catheter to treat chronic total occlusions in angioplasty procedures. The company's mission is to ensure that no patient is left untreated, and has helped physicians save thousands of patients from unnecessary leg amputations.
Watch the GoBack video: https://youtu.be/Rnimv2HGQZk ; www.upstreamperipheral.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220104005157/en/
For more information, contact Avi Posen, VP Sales at avi@UpstreamPeripheral.com.
Source: Upstream Peripheral Technologies
The GoBack Catheter is a single-lumen crossing catheter which features a curved nitinol needle that serves as an effective crossing tool. The versatile needle can be extended straight or to a curved position beyond the GoBack Catheter’s tip. The protrusion length is determined by the clinician with a thumb selector on the device’s handle. The GoBack comes in two configurations of 4 French and 2.9 French for above and below the knee procedures. The GoBack has regulatory approval in 30 countries. (Photo: Upstream Peripheral Technologies)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20220104005157/en