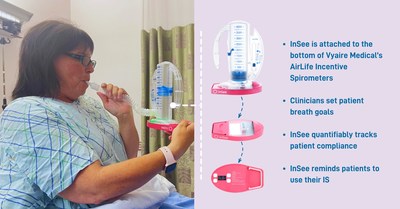
Tidal Medical Technologies today announced that the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for emergency use of the InSee incentive spirometer accessory for quantitatively tracking patient usage of Vyaire Medical’s AirLife incentive spirometer, as an aid in treatment of respiratory conditions by patients with Coronavirus Disease 2019 (COVID-19) in hospital settings.
|
DALLAS, July 14, 2021 /PRNewswire/ -- Tidal Medical Technologies today announced that the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for emergency use of the InSee incentive spirometer accessory for quantitatively tracking patient usage of Vyaire Medical's AirLife incentive spirometer, as an aid in treatment of respiratory conditions by patients with Coronavirus Disease 2019 (COVID-19) in hospital settings. FDA Emergency Use Authorization (EUA) issued for InSee, a patented device for tracking incentive spirometers compliance
In reaching their decision, FDA said there were no approved or cleared devices for quantitatively tracking patient usage of spirometers as an aid in treatment of respiratory conditions by patients with COVID-19 in hospital settings. Incentive spirometers are used during recovery of respiratory illness to strengthen the muscles used for breathing and increase ventilation. Use of InSee in conjunction with an incentive spirometer may assist in treating respiratory conditions and improve clinical outcomes by monitoring and encouraging more frequent and consistent usage of the spirometer, without reliance on the presence of the healthcare provider or reliance on the patient's memory and reporting for use of the spirometer. The FDA's EUA letter further affirms the following about the InSee:
"As we are gearing up for our initial fundraising, we are thrilled that the FDA has recognized the need for quantitative tracking of Respiratory care in hospital setting. Incentive Spirometry has been used widely to help prevent pneumonia in bed-bound patients and help improve lung capacity in Respiratory patients, like COVID-19. With the InSee, hospitals will have objective data and smart-monitoring to better help the prevention and treatment of respiratory conditions." Said Alex Farahmand, MD, CEO of Tidal Medical Technologies. Incentive spirometers are widely used in hospital and Skill Nursing Facilities (SNF) during recovery from surgeries or respiratory illness to strengthen the muscles used for breathing and increase ventilation. The company is in the process of preparing a 510(k) submission to gain concurrence from the FDA for wider use of the InSee so other patients and healthcare practitioners may experience the benefits that this innovative technology has to offer "It always baffles me that we ask patients to use Incentive Spirometers but have no way of knowing if they're being used effectively, if at all. InSee will change that and I'm thrilled that the FDA has recognized InSee's utility." Said Hasan Kakli, MD, Chief Medical Officer at Tidal Medical Technologies. To learn more about InSee, visit TidalMed.Tech To see the FDA EUA, visit https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/respiratory-assist-devices-euas About Tidal Medical Technologies: The company is dedicated to solving the kind of routine challenges that clinicians face every day. In United States, we spend over $10 billion annually on hospital readmissions. Millions of patients are returning to the hospital when they should be at home with their families. While our healthcare system is complex, the reasons for readmissions usually are not. We believe that affordable, common sense solutions can yield tremendous improvements in quality, satisfaction and cost of patient care, also known as the Triple Aim. Connect with us at linkedin.com/company/tidalmed-tech and on Twitter @TidalMedTech Photos available upon request. US Media and Investors Contacts: Mehdi Arani Tidal Medical Technologies
SOURCE Tidal Medical Technologies |