THREAD, a technology and service provider enabling decentralized clinical trials (DCTs), today announced the launch of the industry’s first analytics dashboards designed to specifically monitor, measure and predict DCT performance
|
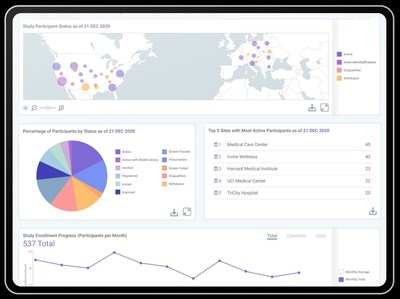
CARY, N.C., May 12, 2021 /PRNewswire/ -- THREAD, a technology and service provider enabling decentralized clinical trials (DCTs), today announced the launch of the industry's first analytics dashboards designed to specifically monitor, measure and predict DCT performance. The analytics dashboard provides researchers with real-time, actionable insights to reduce risk, automate oversight and improve outcomes. THREAD analytics allow study teams and sites to view DCT analytics such as:
Built to address researchers' needs for up-to-the-minute information as they conduct decentralized clinical trials and registries, THREAD's DCT analytics dashboards provide a simple, configurable and actionable approach to performance within the THREAD platform. The combination of descriptive metrics, predictive insights and proprietary algorithms enable research teams to make real-time decisions based on accurate information related to all aspects of their decentralized approach. All study stakeholders can now access data in a single actionable view from all DCT sources including recruitment, CRFs, eSource, eCOA, telehealth, sensors and more. "DCTs require both modified and new performance indicators. As the leader in global decentralized research, we utilized our experience to bring these standard KPIs and DCT-focused insights into our comprehensive platform for our customers," said John Reites, CEO, THREAD. "We are very encouraged with our customers' early adoption and positive feedback on the value of this analytics suite to enable research oversight." THREAD's platform's ability to predict recruitment, enrollment and study completion through its new analytics dashboard will take the guesswork out of these activities. Determining a participant's probability of dropout and seeing which activities are causing compliance issues enable a data-driven approach to achieving study efficiency, providing quality clinical data and retaining participants. Sponsors interested in learning more about the new analytics dashboards visit the THREAD website. About THREAD Media Contact:
SOURCE THREAD |