Gmax Biopharm LLC. announced today that its application of investigation of New Drug (IND) for GMA131 injection, the first global ETa-specific monoclonal antibody, was approved by the US FDA on Nov 11, 2022 for clinical study on diabetic kidney disease (DKD).
|
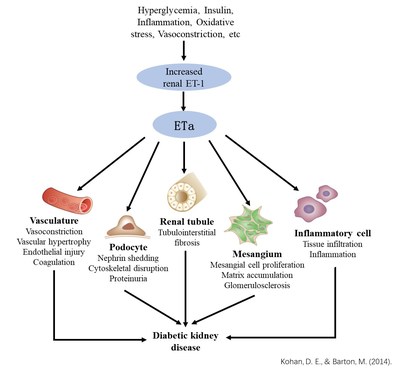
HANGZHOU, China, Nov. 14, 2022 /PRNewswire/ -- Gmax Biopharm LLC. announced today that its application of investigation of New Drug (IND) for GMA131 injection, the first global ETa-specific monoclonal antibody, was approved by the US FDA on Nov 11, 2022 for clinical study on diabetic kidney disease (DKD). Dr. Shuqian Jing, Founder and Chairman of Gmax Biopharm, said, "GMA131 is the first genuine ETa-specific monoclonal antibody developed by Gmax Biopharm. It specifically blocks ET-1/ETa signal transduction with no cross-reactivity with ETb. GMA131 is the same molecule as GMA301 which is currently in a Phase Ib trial of pulmonary arterial hypertension (PAH) in China/USA. Pre-clinical and clinical studies indicate that this molecule demonstrates excellent safety profile with superior efficacy to small molecule endothelin receptor antagonist (ERA) in the high fat diet-STZ induced DKD model. GMA131 is expected to be a new generation drug for DKD." Endothelin (ET-1) and its receptors (ETa and ETb) are widely distributed in the kidney. ET-1/ETa axis regulates renal hemodynamics, function and structural integrity of glomerular filtration barrier, mesangial cell proliferation, extracellular matrix accumulation, inflammation, fibrosis etc., actively contributes to the development and progression of DKD and chronic kidney disease. Meanwhile, ETb plays an important role in the regulation of salt-water homeostasis. Clinical trials of small molecule ERAs have shown that ETa selective antagonists can reduce proteinuria and improve renal function in DKD patients. However, water/sodium retention due to non-specific binding with ETb and the adverse effects due to the specific structure of small molecules are the main obstacles for clinical application of small molecule ERAs. In clinical trials, GMA301, the same molecule as GMA131, has shown superior safety profile in healthy volunteers and pulmonary arterial hypertension patients, without water/sodium retention and edema commonly associated with small molecule ERAs. DKD, the leading cause of chronic kidney disease and end stage renal disease (ESRD), is one of the most important complications of diabetes. About one third of diabetic patients will develop DKD, and will eventually progress to ESRD within about 5 years if presented with massive proteinuria. According to IDF Diabetes Atlas 10th Edition 2021, approximately 537 million adults (20-79 years of age) are living with diabetes worldwide in 2021, about 1 in 10 adults. The numbers are predicted to rise to 783 million (12.2%) in 2045. DKD has become one of the major diseases that seriously endanger human health. As the first ETa-specific monoclonal antibody drug worldwide, GMA131 is poised to offer exceptional benefit to the DKD patients! About Gmax Biopharm LLC: Gmax is a clinical stage biopharmaceutical company established in 2010 and headquartered in Hangzhou, China. It's a global company focusing on R&D, production and commercialization of antibody drugs targeting GPCRs. Gmax mainly works on cardiovascular, metabolic and cancer areas. Currently there are five drug development programs in different stages of clinical trials: GMA 102 (type 2 diabetes), GMA105 (obesity), GMA301A (PAH for adults), GMA301B (PAH for children), GMA106 (obesity/T2DM/NASH). GMA131 (DKD/CKD) is about to enter into phase 1b trial in the USA. Its unique M-body technology empowers acting on two or more different targets to improve drug efficacy or broaden indications. Contact: IR@gmaxbiopharm.com
SOURCE Gmax Biopharm LLC |