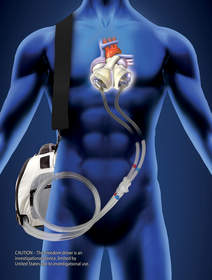
TUCSON, AZ--(Marketwire - April 28, 2011) - On April 13, SynCardia Systems, Inc., manufacturer of the SynCardia temporary Total Artificial Heart, presented awards to seven SynCardia Certified Centers during its Clinical Investigator meeting in San Diego for its FDA-approved Investigational Device Exemption (IDE) clinical study of the Freedom® portable driver. The 13.5-lb Freedom driver, which is also CE approved for use in Europe, is the world's first wearable driver designed to power the Total Artificial Heart both inside and outside the hospital.
Outstanding Outcomes Award
- Recipient:
Dr. Vigneshwar Kasirajan & Team, VCU Medical Center (Richmond, VA) - Achievement:
The team at VCU, one of SynCardia's highest volume centers, saved the lives of 27 consecutive patients dying from end-stage biventricular failure with the Total Artificial Heart. All 27 patients have either been transplanted or are currently awaiting a matching donor heart on the device. VCU currently has five patients supported by the Freedom driver.
Freedom® Pioneer Award
- Recipients:
Dr. Francisco Arabia & Team, Mayo Clinic (Phoenix, AZ)
Pr. Daniel Duveau & Team, University Hospital of Nantes (Nantes, France)
Prof. Pascal Leprince & Team, La Pitié Hospital (Paris, France)
Prof. Jan Gummert & Team, Heart & Diabetes Center NRW (Bad Oeynhausen, Germany) - Achievement:
These hospitals exhibited leadership in use of the Freedom portable driver to discharge stable Total Artificial Heart patients. Mayo Clinic discharged the first Total Artificial Heart patient using the Freedom driver in the U.S. and University Hospital of Nantes, the first in Europe. La Pitié has performed more implants of the Total Artificial Heart than any other center in the world and the Heart & Diabetes Center NRW leads all European centers in Freedom driver use.
Best Launch Award
- Recipients:
U.S. -- INTEGRIS Baptist Medical Center (Oklahoma City, OK)
International -- St. Vincent's Hospital (Sydney, Australia) - Achievement:
INTEGRIS and St. Vincent's were both recognized for their rapid use of SynCardia's Total Artificial Heart and Freedom driver. A month after performing its first implant, INTEGRIS discharged its first patient using the Freedom driver. INTEGRIS performed two more implants soon after. St. Vincent's performed its first two implants within 13 days of each other. Both patients recovered rapidly and were switched to the Freedom driver within two weeks of implant. One patient has since been transplanted and the other is currently waiting for a matching donor heart. St. Vincent's has performed 5 implants overall.
CAUTION -- The Freedom® portable driver is an investigational device, limited by United States law to investigational use.
About the SynCardia temporary Total Artificial Heart
SynCardia Systems, Inc. (Tucson, AZ) is the privately-held manufacturer of the world's first and only FDA, Health Canada and CE approved Total Artificial Heart. Originally used as a permanent replacement heart, the Total is currently approved as a bridge to transplant for people dying from end-stage biventricular heart failure. More than 900 implants account for more than 210 patient years of life.
Similar to a heart transplant, SynCardia's Total Artificial Heart replaces both failing heart ventricles and the four heart valves. It is the only device that eliminates the symptoms and source of end-stage biventricular failure.
In March 2011, Fast Company magazine ranked SynCardia #20 among the World's 50 Most Innovative Companies "for giving mobility to artificial heart recipients."
For additional information, please visit: http://www.syncardia.com
or follow SynCardia on Twitter - @SynCardia_News
Media Contact:
Don Isaacs
Vice President of Communications
SynCardia Systems, Inc.
Cell: (520) 955-0660