CLEVELAND, Oct. 13, 2015 /PRNewswire/ -- Synapse Biomedical Inc. (www.synapsebiomedical.com) reported today that the U.S. multi-center, Post Approval Study (PAS) studying the use of the NeuRx® Diaphragm Pacing System (DPS) in ALS is continuing to enroll patients.
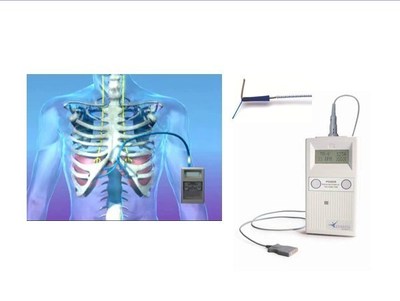
The Post Approval Study (PAS) is being conducted at 11 sites in the United States and patient selection is according to the FDA market-approved criteria. The PAS study group met their initial enrollment goal ahead of schedule in August 2014. The study has since expanded the enrollment target to 150 participants.
"We have performed an interim analysis of the current data (n=60 patients) on the use of NeuRx® Diaphragm Pacing System (DPS) in ALS patients. We see no significant safety issue with the use of the NeuRx DPS® in ALS patients in our PAS study. Furthermore, we interpret the recent UK study as important, but not conclusive. Thus we believe that further study is warranted to determine the portion of the ALS population that will benefit most from the use of the DPS," said Dr. Robert Miller, Principal Investigator of the PAS and Director of CPMC's Forbes Norris MDA/ALS Research and Treatment Center in San Francisco.
In fact, the interim analysis of the first 60 study patients shows a median survival of 20.9 months post-implant. These results are consistent with the survival demonstrated in the original IDE pivotal study (106 patients; 8 centers) which reported a median survival of 19.7 months after implant. Dr. Miller will be presenting the PAS interim analysis at the 26th International Symposium on ALS/MND to be held in Orlando, US from 11-15 December.
"We are committed to ongoing clinical research studies, such as the PAS, to further clarify the safety and benefit of diaphragm pacing for people with ALS," said Mike Fritz, VP Clinical and Regulatory Affairs for Synapse Biomedical.
About NeuRx DPS®Technology
NeuRx DPS® (NeuRx DPS®) is a four-channel, battery-powered neurostimulator with implanted electrodes. The device provides electrical stimulation to the muscle and nerves of the diaphragm. The NeuRx DPS® received CE Marking (CE Registration #518356) on November 20, 2007 and is approved for treating patients with diaphragm dysfunction in the European Union. The NeuRx DPS® received FDA approval for ventilator dependency from spinal cord injury on June 17, 2008. In Spinal Cord Injury (SCI), the NeuRx DPS® provides ventilator support in patients with diaphragm dysfunction of neuromuscular origin. Diaphragm dysfunction can result in abnormal or absent respiration in patient populations of high-level spinal cord injury and other injuries or diseases affecting the neuromuscular respiratory pathways. The NeuRx DPS® received FDA approval for treating chronic hypoventilation from ALS on September 28, 2011. The NeuRx DPS® has demonstrated that it can help people with ALS live longer and sleep better than the current standard of care alone.
For more information please visit www.synapsebiomedical.com/products/patientInfo.shtml

Photo - http://photos.prnewswire.com/prnh/20151013/276436
Logo - http://photos.prnewswire.com/prnh/20131113/CL14319LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/us-diaphragm-pacing-study-in-als-continues-enrollment--the-post-approval-studys-pas-interim-data-showed-device-safety-and-median-survival-post-implant-of-209-months-300158826.html
SOURCE Synapse Biomedical Inc.
 Help employers find you! Check out all the jobs and post your resume.
Help employers find you! Check out all the jobs and post your resume.




