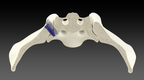
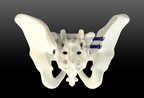
A leader in innovative medical devices, is proud to announce clearance from the U.S. Food and Drug Administration for their proprietary TiLink-L Sacroiliac Joint Fusion System.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230608005677/en/

(Graphic: Business Wire)
TiLink-L stands out in the market due to its distinct features. Its intuitive design allows for superior compression across the joint, offering patients an exceptional platform for fusion. In addition, the implant is equipped with SurGenTec’s proprietary Nanotex® surface technology. In-vivo studies conducted by a Good Laboratory Practice Facility demonstrated bony in-growth and on-growth which may enhance stability for improved surgical outcomes. The product also boasts a helical self-harvesting channel to capture the patient’s own bone and encourage healing. Graft windows are strategically placed between threads to allow for potential fusion through the screw to enhance stability. Complementing these essential features is a comprehensive variety of implant lengths, ensuring a custom fit for patients, thereby providing optimal fixation, and reducing potential complications.
SurGenTec’s new product enters an emerging market that has seen significant growth. According to a recent report, the sacroiliac joint fusion market is projected to witness an exponential rise in the upcoming years. As the population ages and the demand for minimally invasive surgical solutions increases, SurGenTec’s TiLink-L implant is strategically positioned to address this burgeoning need.
"We are thrilled to announce the FDA clearance of our first treatment option in our sacroiliac family of products,” says Travis Greenhalgh, CEO at SurGenTec. "With its unique properties and ability to adapt to diverse patient needs, TiLink-L is set to offer physicians the ability to stabilize the sacroiliac joint in a variety of approaches. Whether in an outpatient or hospital setting, we believe this device is poised to make a significant difference in patient care.”
SurGenTec’s commitment to developing cutting-edge medical devices is further solidified with this latest release. TiLink-L reaffirms the company’s dedication to improving patient outcomes and revolutionizing the SI joint fusion market.
SurGenTec is a privately owned medical device company based in Boca Raton, Florida that is focused on unique technologies to improve orthopedic and spine care. SurGenTec develops and manufactures innovative products with patient and surgeon safety in mind. SurGenTec looks to launch TiLink-L immediately in the United States. The vast pipeline of unique implants, instruments, biologics, and ancillary solutions will continue to develop through a high level of internal research and development. SurGenTec currently has an array of products to market which are sold within the United States and internationally. Multiple emerging technologies will continue to be released throughout the remainder of 2023.
For more information on TiLink-L, visit www.SurGenTec.com or contact customerservice@surgentec.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230608005677/en/
Contacts
Ricki Goldman
SurGenTec
+1 561-990-7882
Email us here:
customerservice@surgentec.com
Visit us on social media:
https://www.linkedin.com/in/surgentec/
Source: SurGenTec