Sun Pharmaceutical Industries, Inc., a wholly owned subsidiary of Sun Pharmaceutical Industries Ltd. is voluntarily recalling one lot of RIOMET ER™, 500 mg per 5 mL to the consumer level.
PRINCETON, N.J.--(BUSINESS WIRE)-- Sun Pharmaceutical Industries, Inc. (SUN PHARMA), a wholly owned subsidiary of Sun Pharmaceutical Industries Ltd. is voluntarily recalling one lot of RIOMET ER™ (metformin hydrochloride for extended-release oral suspension), 500 mg per 5 mL to the consumer level. The reason for the recall is due to the level of N-Nitrosodimethylamine (NDMA), which has been found to be above the allowable Acceptable Daily Intake (ADI) limit established by the U.S. Food and Drug Administration.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200923005168/en/
(Photo: Business Wire)
NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. To date, SUN PHARMA has not received any reports of adverse events related to this recall.
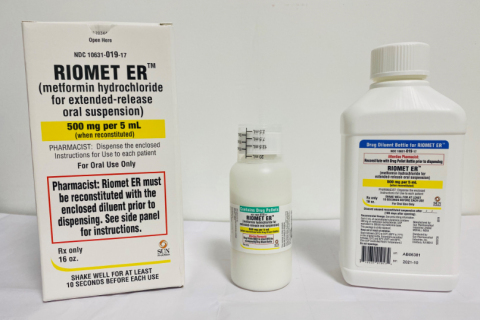
RIOMET ER™ (metformin hydrochloride for extended-release oral suspension) is a prescription oral medication indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus. RIOMET ER™, when reconstituted, is packaged in a 16 oz. (473 mL) round bottle. Each carton contains one bottle of drug pellets, one bottle of diluent, and one dosing cup. The affected RIOMET ER™ is the following lot:
|
Product Name |
Lot |
NDC Number |
Expiration |
Number |
|
RIOMET ER™ (metformin hydrochloride for extended-release oral suspension), 500 mg per 5 mL |
AB06381 |
10631-019-17 |
10/2021 |
747 cartons |
The product can be identified by the bottles or carton labeled as RIOMET ER™ (metformin hydrochloride for extended-release oral suspension), containing the specific Lot Number and Expiration Date referenced above or on the labeling below. The product was distributed nationwide to wholesale customers.
SUN PHARMA is notifying its distributors and customers through its third party Recall Coordinator (Inmar Inc.), via FedEx standard overnight shipping and will arrange for return of all recalled products.
Distributors and retailers that have RIOMET ER™ (metformin hydrochloride for extended-release oral suspension), which is being recalled, should stop distributing and return it to place of purchase or as directed in the recall notification.
Patients taking RIOMET ER™ (metformin hydrochloride for extended-release oral suspension), 500 mg per 5 mL are advised to continue taking their medication and contact their pharmacist, physician, or medical provider for advice regarding an alternative treatment. According to the U.S. Food & Drug Administration, it could be dangerous for patients with this serious condition to stop taking their metformin without first talking to their health care professionals. Please visit the agency’s website for more information at https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-metformin.
Consumers with questions regarding this recall can contact SUN PHARMA by calling 1-800-818-4555 Monday through Friday between 8:00 am to 5:00 pm EST or e-mailing drug.safetyUSA@sunpharma.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
CORP-US-RER-0001
View source version on businesswire.com: https://www.businesswire.com/news/home/20200923005168/en/
Company Contacts:
Consumers
Drug.safetyUSA@sunpharma.com
1-800-818-4555
Media
Vinita Alexander
vinita.alexander@sunpharma.com
1-609-720-8197
Source: Sun Pharmaceutical Industries, Inc.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20200923005168/en







