PARIS, April 28, 2017 /PRNewswire/ -- Sanofi (NYSE: SNY; EURONEXT: SAN)
Q1 2017 | Change | Change at CER | Change at CER and CS(1) | |
Business net income(2) | 1,795m | +4.2% | +1.0% | - |
Business EPS(2) | 1.42 | +6.0% | +3.0% | - |
Experience the interactive Multimedia News Release here: https://www.multivu.com/players/English/8093351-sanofi-earnings-results-q1-2017/
First-quarter 2017 accounts reflect the acquisition of the former Boehringer Ingelheim Consumer Healthcare (CHC) business and the disposal of the Animal Health business (completed on January 1, 2017(3)). In accordance with IFRS 5 (Non-Current Assets Held for Sale and Discontinued Operations), Animal Health results in 2016 and gain on disposal in 2017 are reported separately. The first quarter 2017 income statement also reflects the consolidation of European operations related to Sanofi vaccine portfolio, following the termination of the Sanofi Pasteur MSD joint venture (SPMSD JV) with Merck at the end of December 2016. |
(1) CS: constant structure: adjusted for BI CHC business, termination of SPMSD and others; (2) In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income is a non-GAAP financial measure (see Appendix 8for definitions). The consolidated income statement for Q1 2017 is provided in Appendix 3 and a reconciliation of IFRS net income reported to business net income is set forth in Appendix 4; (3) The closing of the disposal of Merial in Mexico is expected in 2017
Sanofi Chief Executive Officer, Olivier Brandicourt, commented:
"We have started the year with robust growth driven by Specialty Care and Vaccines as well as good performance in Emerging Markets. Our top line in the first quarter also benefited from the integration of the Boehringer Ingelheim CHC and European vaccine businesses. At the same time, the simplified organization continues to contribute to Sanofi's financial performance. The U.S. launch of Dupixent® for moderate-to-severe atopic dermatitis marks a key innovation milestone on our strategic roadmap and lays the foundation for our new immunology franchise. We are excited to bring this highly innovative medicine to patients suffering from this devastating disease."
Q1 2017 sales growth supported by Specialty Care, Vaccines and Emerging Markets
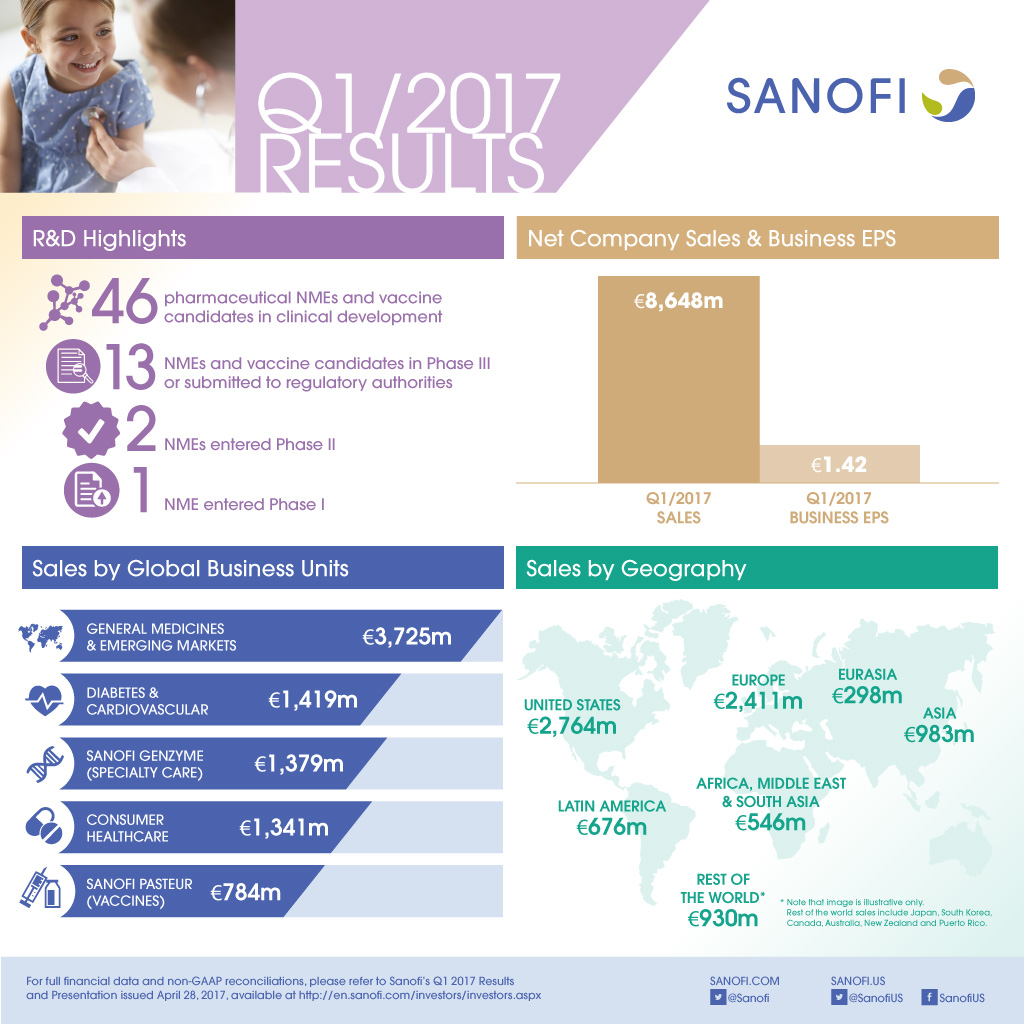
- Net sales were 8,648 million, up 11.1% on a reported basis and 8.6% at CER reflecting the acquisition of Boehringer Ingelheim's (BI) CHC business and full consolidation of Sanofi's European vaccine operations. At constant structure and CER, net sales were up 3.5%.
- Sanofi Genzyme (Specialty Care) GBU sales increased 15.5% at CER driven by Multiple Sclerosis products.
- Diabetes and Cardiovascular GBU sales were down 7.7% at CER; Global Diabetes franchise sales decreased 6.0%.
- Sanofi Pasteur GBU grew 13.2% at CER and constant structure due to the strong performance of pediatric combinations.
- CHC GBU sales were up 4.7% at CER and constant structure driven by the performance in Europe.
- Emerging Markets sales increased 8.5% at CER and constant structure.
Strong financial results and 2017 guidance confirmed
- Business operating income of 2,442 million, up 7.6% at CER and constant structure.
- Business EPS grew 3.0% at CER to 1.42 and increased 6.0% on a reported basis.
- Sanofi continues to expect 2017 Business EPS to be stable to -3% at CER, barring unforeseen major adverse events.
- IFRS net income of 5,701 million (up 424%) included a net gain of 4,427 million resulting from the divestment of Merial.
Sanofi progresses on its 2020 roadmap
- Integration of Boehringer Ingelheim CHC business on track, enhancing Sanofi's position in key categories and regions.
- Following the termination of the SPMSD JV, European vaccine business now fully driven by Sanofi.
- Dupixent®, a breakthrough therapy for moderate-to-severe atopic dermatitis, now available to adult patients in the U.S.
- Soliqua100/33, first once-daily fixed combination of Lantus® and lixisenatide for type-2 diabetes, launched in the U.S.
- Kevzara BLA for the treatment of rheumatoid arthritis granted PDUFA date of May 22, 2017.
- FDA approval of Xyzal® Allergy 24H for OTC use and launch underway ahead of the U.S. spring allergy season.
R&D update
Regulatory update
Regulatory updates since the publication of 2016 full-year results on February 8, 2017 include the following:
- In April, the FDA approved a new dosing regimen for Praluent® of 300 mg administered subcutaneously once monthly (every 4 weeks).
- In April the European Medicine Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) granted a positive opinion for the marketing authorization of Kevzara® (sarilumab), recommending its approval for use in adult patients with moderately to severely active rheumatoid arthritis.
- In March, the U.S. Food and Drug Administration (FDA) approved Dupixent® (dupilumab), the first and only biologic medicine approved for the treatment of adults with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
- Following successful conclusion of Le Trait manufacturing site inspection by FDA, the Kevzara (sarilumab) U.S. BLA was accepted in April for the treatment of rheumatoid arthritis with a PDUFA date of May 22, 2017.
- At the end of April 2017, the R&D pipeline contained 46 pharmaceutical new molecular entities (excluding Life Cycle Management) and vaccine candidates in clinical development of which 13 are in Phase 3 or have been submitted to the regulatory authorities for approval.
Portfolio update
Phase 4:
- Top-line results of the ODYSSEY OUTCOMES study on Praluent® are now expected to be reported in the first quarter of 2018 based on communications from the independent DSMB (Data and Safety Monitoring Board). Recruitment for this 18,600-patient cardiovascular outcomes trial was completed in November 2015 and the scheduled two-year follow-up of patients is underway.
Phase 3:
- The results of the CAFÉ study evaluating dupilumab in cyclosporine-resistant patients in moderate-to-severe atopic dermatitis were positive and demonstrated an acceptable safety profile. These results will be submitted to the EMA and presented at a scientific Congress.
- In March, detailed results from the one-year Phase 3 CHRONOS study were presented at the Annual Meeting of the American Academy of Dermatology (AAD). In this study, patients receiving Dupixent® with topical corticosteroids (TCS) achieved significantly improved measures of overall disease severity compared to TCS alone in adults with uncontrolled moderate-to-severe AD with a safety profile consistent with previous studies.
Phase 2:
- SP0232 / MEDI8897 (partnership with MedImmune), a monoclonal antibody, entered the portfolio in Phase 2 for the prevention of lower respiratory tract illness in infants caused by respiratory syncytial virus.
- SAR566658, a maytansin-loaded anti-CA6 monoclonal antibody, entered into Phase 2 for the treatment of triple negative breast cancer.
- A Phase 2 study was initiated to evaluate isatuximab in acute lymphoblastic leukemia.
Phase 1:
- SAR440181 / MYK491 (collaboration with MyoKardia), for the treatment of dilated cardiomyopathy (DCM1 myosin activation), entered Phase 1.
To access the full press release of the 2017 Q1 results, please click here.
2017 guidance
Sanofi expects 2017 Business EPS to be stable to -3% at CER, barring unforeseen major adverse events, consistent with its previously announced Strategic Roadmap guidance for the 2016-17 period. Applying the average March 2017 exchange rates to the rest of the year, the currency impact on 2017 Business EPS is estimated to be +3% to +4%.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, Sanofi's ability to benefit from external growth opportunities and/or obtain regulatory clearances, risks associated with intellectual property and any related pending or future litigation and the ultimate outcome of such litigation, trends in exchange rates and prevailing interest rates, volatile economic conditions, the impact of cost containment initiatives and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2016. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
Media Relations:
Ashleigh Koss
908-981-8745
Email: Ashleigh.Koss@sanofi.com
Investor Relations:
George Grofik
+33 (0)1 53 77 45 45
Email: IR@sanofi.com




To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/sanofi-delivers-robust-q1-2017-financial-results-300447941.html
SOURCE Sanofi





