RhythMedix, a leader in remote cardiac monitoring and proprietary arrhythmia detection algorithms, today announced expanded modalities for its RhythmStar® System (RhythmStar).
|
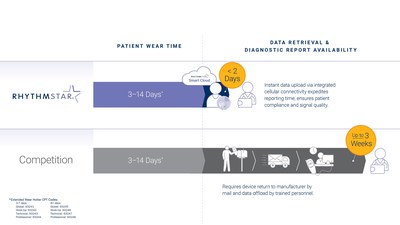
Strategic addition of Extended Wear Holter monitoring modality enhances portfolio offering and positions company for accelerated growth MOUNT LAUREL, N.J., Oct. 18, 2022 /PRNewswire/ -- RhythMedix, a leader in remote cardiac monitoring and proprietary arrhythmia detection algorithms, today announced expanded modalities for its RhythmStar® System (RhythmStar). The addition of long-term continuous electrocardiogram (ECG) monitoring up to 14 days provides clinicians with a complete and flexible cardiac monitoring solution, with rapid report turnaround in less than 48 hours, to address a diverse range of patient needs. "We are especially pleased to launch expanded capabilities so close to National AFib Awareness Month. Early arrhythmia detection is critical. RhythMedix is committed to transforming and simplifying the diagnostic pathway to enable faster diagnosis, without missing a beat," stated Brian Pike, President, of RhythMedix. "We've monitored over 1 million patients and look forward to accelerating adoption of our innovative remote cardiac monitoring solution to reach even more patients." Alternative monitoring systems require patients to send the wearable device back to the manufacturer for manual data retrieval, analysis and report generation. Unlike these systems, RhythmStar instantly transfers data wirelessly to enable faster ECG analysis with rapid report turnaround, so that care providers receive diagnostic reporting weeks faster than with mail back data retrieval methods. The RhythmStar platform fits seamlessly into clinical practice and its interoperability support easily integrates with electronic medical record (EMR) systems. In addition to the new Extended Wear Holter option, RhythMedix offers multiple modalities including mobile cardiac telemetry. Heart arrhythmias are a major health concern and double the risk of death in an estimated 37.5 million people worldwide. Atrial fibrillation (AFib) is the most frequent cardiac arrhythmia and a major risk factor for ischemic stroke.1,2 While remote cardiac monitoring has improved arrhythmia detection, patient compliance and timely access to heart activity remain a challenge with currently available competitive monitoring technologies. Since the launch of its next-generation RhythmStar System last year, the company is experiencing tremendous near 30% year-over-year growth and has more than doubled adoption of its platform by cardiologists, electrophysiologists, and healthcare systems across the United States. The sleek, patient-friendly device is the first one-piece, wearable cardiac monitor with built-in cellular connectivity. This novel feature enables near real-time arrhythmia verification without the need for a separate communication device, making it simpler for the patient and clinician while maximizing patient compliance. Follow RhythMedix on LinkedIn, Facebook and Twitter for the latest news. ABOUT RHYTHMEDIX: RhythMedix®, a privately owned medical device manufacturer and wireless medical technology company, provides innovative technology to transform the cardiac monitoring industry and improve patient outcomes. RhythmStar® is the first wearable cardiac monitor with built-in cellular connectivity, allowing physicians and their patients to remain continuously and comfortably connected for faster arrhythmia detection. Powerful cloud-based algorithms evaluate every heartbeat providing actionable reporting that is verified by the RhythMedix Independent Diagnostic Testing Facility (IDTF). Learn more at www.rhythmedix.com.
SOURCE RHYTHMEDIX/ SPRIG CONSULTING, LLC |