Researchers with the NYU School of Medicine and the University of Michigan identified a gene called ATDC that is necessary for the development of pancreatic cancer. They published their research in the journal Genes & Development.
Originally published on May 3, 2019.
Researchers with the NYU School of Medicine and the University of Michigan identified a gene called ATDC that is necessary for the development of pancreatic cancer. They published their research in the journal Genes & Development.
PDA is an aggressive form of pancreatic cancer driven by the oncogene KRAS. It is usually diagnosed late and is resistant to therapy.
There is a theory that many cancers develop when adult cells shift back into more “primitive” cells that have higher growth in order to resupply cells lost to injury and inflammation. When this shift occurs with other genetic errors, a repair process that is supposed to start and stop growth stops working.
Their research found that a gene called ATDC is necessary for pancreatic cancer to form.
“We found that deleting the ATDC gene in pancreatic cells resulted in one of the most profound blocks of tumor formation ever observed in a well-known mice model engineered to develop pancreatic ductal adenocarcinoma, or PDA, which faithfully mimics the human disease,” stated Diane Simeone, director of the Pancreatic Cancer Center of NYU Langone Health’s Perlmutter Cancer Center, and corresponding author of the study. “We thought the deletion would slow cancer growth, not completely prevent it.”
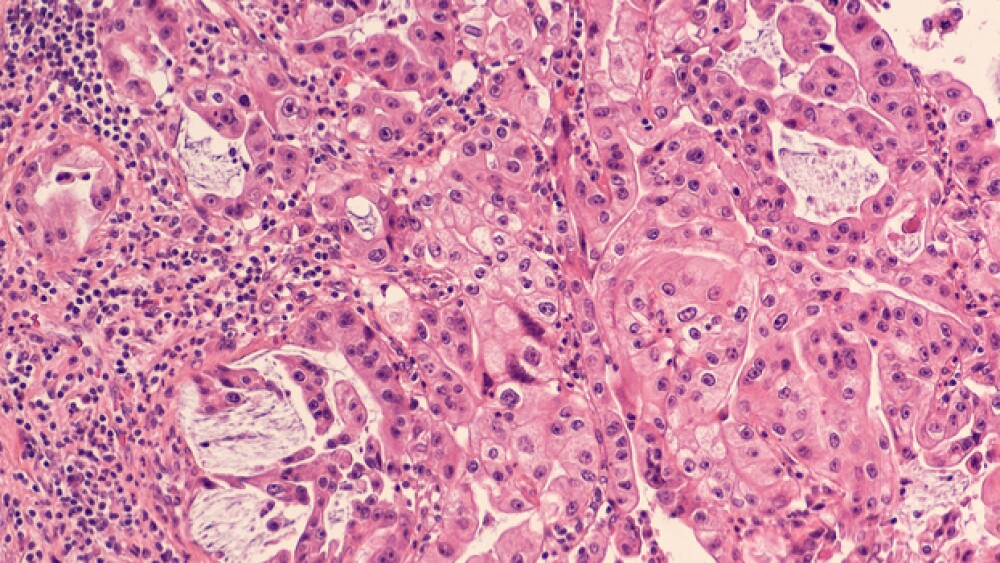
Their research focused on acinar cells in the pancreas. These cells secrete digestive enzymes via a network of partnering ducts into the small intestine. The digestive enzymes can cause low-level damage to tissues. Acinar cells respond by quickly switching back into stem cell types that resemble high-growth primitive cells, which is also common in pancreatic duct cells.
The ability to regenerate comes with a problem, though, which they are more likely to become cancerous, including those in the KRAS gene which is linked to aggressive growth in more than 90% of pancreatic cancers.
Stressed acinar cells temporarily shift from acinar-to-ductal metaplasia (ADM), a step toward the primitive cell type. This creates an environment for a second shift into pancreatic intraepithelial neoplasia (PanIN). These cells can’t multiply under normal controls.
The team found that mutant KRAS and other genetic mutations induced aggressive pancreatic cancer in 100% of the mice when the ATDC gene was present. However, there was no cancer in the same cancer-prone mice who lacked the gene.
The group created pancreatitis in the mice by treating them with cerulein, a signaling protein fragment that damages pancreatic tissues. They found that ATDC gene expression didn’t immediately increase after the damage, but took a few days, in keeping with the timeframe associated with acinar cells being reprogrammed into their ductal cell-type cells.
They further found that the expression of ATDC triggered beta-catenin, a cell-signaling protein that under some circumstances activates genes that include SOX9. SOX9 has been linked to the development of ductal stem cells and to aggressive growth observed in pancreatic cancer.
In addition to the mouse models, the researchers studied ATDC expression in ADM lesions from 12 human pancreatic tissue samples. ATDC was more active in human ADM lesions as well as beta-catenin and SOX9. And the further along during the transition of ADM into human pancreatic ductal adenocarcinoma, the stronger the activation.
The authors state, “These results provide new insight into PDA initiation and reveal ATDC as a potential target for preventing early tumor-initiating events.”