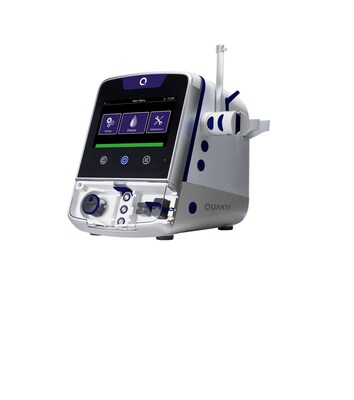
Quanta Dialysis Technologies®, a medical technology company committed to making kidney care more accessible, today announced that it has submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for indication expansion of the Quanta™ Dialysis System, a compact and easy-to-use hemodialysis device.
|
|
| [19-September-2023] |
|
New expanded indication will increase home hemodialysis device options available to patients with end-stage kidney disease BEVERLY, Mass., Sept. 19, 2023 /PRNewswire/ -- Quanta Dialysis Technologies®, a medical technology company committed to making kidney care more accessible, today announced that it has submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for indication expansion of the Quanta™ Dialysis System, a compact and easy-to-use hemodialysis device. The Quanta Dialysis System is currently indicated for use in chronic and acute care settings. The new submission seeks to expand use to include self-care, in-home hemodialysis. According to the U.S. Renal Data System, there are over 490,000 people in the U.S. who use hemodialysis to treat their end-stage kidney disease (ESKD). Home hemodialysis (HHD), which replaces the thrice-weekly commute to a dialysis clinic to receive treatment, accounts for 2% of the total hemodialysis population.i Studies suggest that HHD has many benefits over traditional in-center dialysis such as improvements to quality of life, health outcomes and access to care. In response to the benefits of HHD, the Centers for Medicare and Medicaid Services (CMS) established a program to encourage greater use of home dialysis and kidney transplants for Medicare beneficiaries.ii "The FDA submission for in-home use of the Quanta Dialysis System represents a significant milestone for Quanta in our journey to make dialysis more accessible to every patient in every setting," said Alejandro Galindo, Quanta Chief Executive Officer. "The Quanta Dialysis System is already providing patients in the U.K. with the freedom and flexibility that HHD offers. With clearance of this 510(k), patients in the U.S. will have more control over their care, and choice in where, when and how they receive treatment." This submission, pending regulatory clearance from the FDA, would make the Quanta Dialysis System the third device in the United States indicated for HHD in patients with end-stage kidney disease (ESKD). FDA clearance for the expanded indication is expected in 2024. Quanta's 510(k) submission is supported by clinical data from its Home Run™ study, a prospective, multi-center, open-label trial to assess the efficacy and safety of the Quanta Dialysis System for HHD. Full results from the study will be shared this November during a poster presentation at the upcoming American Society of Nephrology (ASN) Kidney Week in Philadelphia, Pennsylvania. About End-Stage Kidney Disease About Quanta Dialysis Technologies The Quanta Dialysis System is commercially available in the United Kingdom for home and hospital use. In the United States, it is FDA-cleared (K222067) for use in chronic and acute care settings, with treatment types including intermittent hemodialysis (IHD), sustained low efficiency dialysis (SLED), prolonged intermittent renal replacement therapy (PIRRT), slow continuous ultrafiltration (SCUF), and continuous venovenous hemodialysis (CVVHD). To learn more about Quanta and its products, visit quantadt.com. Media Contact: i United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2022.
SOURCE Quanta Dialysis Technologies |