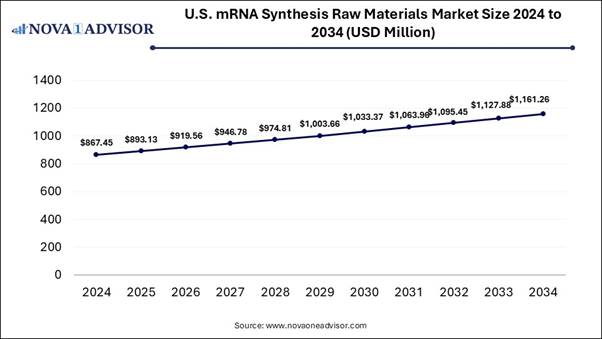
According to Nova One Advisor, the U.S. mRNA synthesis raw materials market size is expected to be worth around 1,161.26 million by 2034, increasing from USD 893.13 million in 2025, representing a healthy CAGR of 2.96% from 2025 to 2034.
The U.S. mRNA synthesis raw materials market is expanding due to mRNA synthesis is important because it provides the blueprint for protein synthesis. Without mRNA, proteins would never be shaped through the process of protein synthesis. The mRNA carries the message from the DNA to the ribosomes.
Successful large-scale mRNA synthesis requires raw material, which includes an RNA polymerase, nucleotides, and a linear DNA template. Furthermore, enzymes such as restriction endonucleases, RNase inhibitors, pyrophosphatases, and DNase I are also used.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/8534
U.S. mRNA Synthesis Raw Materials Market Highlights:
• Nucleotide held the largest revenue market share of 42% in 2024 and is expected to grow at the highest CAGR over the forecast period.
• The enzyme segment is expected to register a significant CAGR over the forecast period.
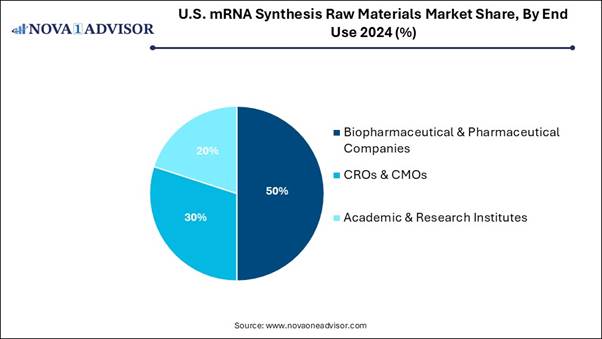
• Biopharmaceutical & pharmaceutical companies dominated the segment with a market share of 50% in 2024.
• The CROs & CMOs segment is expected to grow at a significant CAGR over the forecast period.
• Vaccine production dominated the segment with a revenue market share of 84% in 2024
• The therapeutics segment is expected to grow at a significant CAGR over the forecast period.
Market Overview and Industry Potential:
The U.S. mRNA synthesis raw materials market is expanding rapidly due to the U.S.'s increasing demand for mRNA-based vaccines and therapeutics, as mRNA vaccines are applied to control the spread of numerous life-threatening diseases, like infectious diseases and cancer, and this type of vaccine also applies to immunotherapies. The mRNA vaccines represent a novel class of vaccines that attach the body’s cellular machinery to produce particular antigens, which trigger the immune response against the produced antigens. This novel approach provides significant advantages over traditional vaccine platforms.
Majorly Used Bioprocesses for mRNA Synthesis
|
Enzyme or protein |
Purpose |
|
Restriction endonucleases |
Linearization of plasmid DNA |
|
RNA polymerases |
Synthesis of RNA from linear template DNA
|
|
DNA polymerases |
Synthesis of linear DNA by PCR or isothermal amplification |
|
Pyrophosphatases |
Removal of byproducts from RNA synthesis |
|
RNase inhibitors |
Prevention of RNA degradation |
|
DNase I |
Removal of template DNA |
|
Capping enzymes |
Capping of synthesized RNA at the 5′ end with an m7G analog |
|
2′-O-methyltransferases |
Methylation of capped RNA |
|
Poly(A) polymerases |
Tailing of synthesized RNA at the 3′ end with adenosines |
The therapeutic use of messenger RNA
has driven great hope to fight a broad range of hopeless diseases. Recent rapid
advances in biotechnology and molecular medicine have allowed the production of
almost any functional protein in the human body through introducing mRNA as a
vaccine or therapeutic agent. This signifies an increasing precision medicine
field with great promise for avoiding and treating many intractable or rare
diseases. ⬥︎ For Instance, In May 2025, the mRNA TT
programme had 15 partners, the hub, and 14 manufacturing partners, with
participation still expanding. It is working to expand research and development
(R&D) collaboration and networks, to establish R&D consortia to address
regional and local priority diseases. Latest Trends of the Market ⬥︎ In May 2025, TriLink BioTechnologies, a
Maravai LifeSciences company and global provider of life science reagents and
services, and the International Vaccine Institute (IVI), a non-profit
international organization devoted to and the discovery, development and
delivery of vaccines for global health signed a Memorandum of Understanding to
collaborate to advance the research and development of mRNA-based vaccines and
promote equitable access to essential vaccines and health technologies. ⬥︎ In August 2024, the U.S. Food and Drug
Administration approved and granted emergency use authorization (EUA) for
updated mRNA COVID-19 vaccines (2024-2025 formula) to include a monovalent
component that corresponds to the Omicron variant KP.2 strain of SARS-CoV-2.
The mRNA COVID-19 vaccines have been updated with this formula to more closely
target currently circulating variants and provide better protection against
serious consequences of COVID-19, including hospitalization and death. Increasing Demand for Biopharma:
Market’s Largest Potential Expansion of mRNA therapies pipelines due
to major U.S. companies spending in mRNA drugs as mRNA has huge potential to
prevent and treat a broad range of diseases, and as a breakthrough technology
platform, is expected to moderately replace outdated vaccines and drugs,
creating novel therapeutic opportunities and revolutionizing treatments.
mRNA-driven therapies are renovating the landscape of cancer treatment by
providing a wide range of strategies from vaccines to cell-based therapies,
each with unique mechanisms to tackle different aspects of tumor biology. The
mRNA therapy oncology pipeline is increasing by around 50% due to mRNA vaccines
because of their relatively straightforward production and wide applicability.
Companies such as Moderna, BioNTech, and CureVac are driving this field, with
several vaccines in modern stages of development. ⬥︎ For Instance, In June 2025, BioNTech
SE and CureVac N.V. announced that they have entered into a definitive Purchase
Agreement under which BioNTech intends to acquire all of the shares of CureVac,
a clinical-stage biotech company developing a novel class of transformative
medicines in oncology and infectious diseases based on messenger ribonucleic
acid (“mRNA”). Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8534
Report Scope of U.S. mRNA Synthesis Raw
Materials Market Report Coverage Details Market Size in 2025 USD 893.13 Million Market Size by 2034 USD 1,161.26 Million Growth Rate From 2025
to 2034 CAGR of 2.96% Base Year 2024 Forecast Period 2025-2034 Segments Covered Type, Application,
End-use Market Analysis
(Terms Used) Value (US$
Million/Billion) or (Volume/Units) Regional Scope U.S. Key Companies
Profiled F. Hoffmann-La Roche
Ltd.; Jena Bioscience GmbH; Merck KGaA; Yeasen Biotechnology (Shanghai) Co.,
Ltd.; BOC Sciences; Thermo Fisher Scientific, Inc.; Maravai LifeSciences; New
England Biolabs; Creative Biogene; HONGENE; Evonik Industries AG
U.S. mRNA Synthesis Raw Materials Market
Segmentation Analysis: By Type Analysis: The nucleotides
segment dominated in the U.S. mRNA synthesis raw materials market, as
nucleotides are significant for energy production and gene activation in cells.
Nucleotides, as they offer the structure map for protein synthesis on RNA
sequences, are significant for muscle building. The primary energy transfer
molecule in cells, Adenosine triphosphate (#ATP), is one of the nucleotides.
The integration of modified nucleosides in mRNA has major advantages and is
presently undergoing a renaissance in the field of therapeutic protein
delivery. On the other hand, the enzymes segment is
expected to grow significantly during the forecast period as enzyme suppliers
have a significant impact on consistency, quality, scalability, and government
approval of mRNA production. These enzymes should not only maintain functional
reactions but also be free of contaminants, like host and foreign nucleic
acids, nucleases, and proteins, to help lower challenges and enhance the
effectiveness of mRNA vaccines and therapeutics. By End-use Analysis: The biopharmaceutical
and pharmaceutical
companies segment dominated the U.S. mRNA synthesis raw materials market, as
mRNA products have relatively huge transfection efficiency and reduced toxicity
as they do not require entering the nucleus to be functional. Significantly,
mRNA does not have potential challenges of accidental infection or
opportunistic insertional mutagenesis, so it is widely used by
biopharmaceutical and pharmaceutical companies. The contract
research organizations (CROs) and contract manufacturing organizations
(CMOs) segment is expected to grow significantly during the forecast period, as
dedicated contract research organizations (#CRO) and CMOs with a track record
of support for innovative mRNA products with an experienced and forward-looking
mindset. They are efficient guides for mRNA vaccine and drug developers and
regulatory leaders. By Application Analysis: The vaccine production segment captured the
largest market share in 2024, as mRNA vaccines have a huge success rate in
avoiding severe hospitalization, illness, and death. They do not need the
growth of live virus cultures within a laboratory, a time-consuming process.
These vaccines were developed with much greater efficacy than outdated
vaccines. The mRNA vaccines are designed and synthesized; it quickly
mass-produced. mRNA technology enables rapid vaccine progress. Scientists
rapidly inform the vaccine formula to target novel variants. The therapeutics production segment is expected
to show the fastest growth during the forecast period, as mRNA has huge
flexibility as a technology that allows the production of various drug
categories, with vaccines targeting bacterial, viral, cancer-specific, and
self-antigens; therapeutic antibodies; and protein replacement therapies for
interventional and maintenance treatments. Therapeutic application of mRNA is
the usage of cells as factories to manufacture functional proteins for
protein-replacement therapies. Country Level Analysis: In the U.S., messenger RNA-based therapy
has transformed cancer research by enabling versatile delivery systems for
therapeutic applications. An advanced U.S. startup environment, containing a
vibrant venture capital sector that offers the early-stage funding small
companies require to further develop novel medical technologies and a support
system of specialized vendors and service providers. Continuous and significant
public spending for primary medical research at the NIH and other related
agencies, as well as at U.S. research centers and universities. Growing
healthcare technology investment such as In January 2025, U.S. President Donald
Trump announced a private sector investment of up to $500 billion to fund
infrastructure for artificial intelligence, aiming to outpace rival nations in
business-critical technology. ⬥︎ For Instance, In July 2025, Moderna
announced promising efficacy results from mRNA flu vaccine trial. The company
showed its candidate flu vaccine, called mRNA 1010, showed superior results, with
an rVE of 26.6% in the overall study population (95% confidence interval, 16.7%
to 35.4%). U.S. mRNA Synthesis Raw Materials Market
Companies: • F. Hoffmann-La Roche Ltd. • Yeasen Biotechnology (Shanghai) Co., Ltd. • Thermo Fisher
Scientific, Inc. • New England Biolabs • Creative Biogene • HONGENE • Evonik Industries AG • GENEVANT SCIENCES CORPORATION What is Going Around the Globe? ⬥︎ In January 2025, Moderna, Inc. announced business
updates and progress across its pipeline of mRNA medicines. Moderna enters 2025
with a focus on a prioritized portfolio addressing respiratory viruses, rare
diseases, oncology, and latent and other viruses where there is unmet need. It achieved
$3.0 - 3.1 billion in product sales, approval of our RSV vaccine, and continued
to adapt our COVID-19 business for the endemic setting. ⬥︎ In May 2025, Pfizer Inc. and BioNTech SE announced
that they have submitted a regulatory application to the European Medicines
Agency (EMA) for approval of COMIRNATY for the 2025-2026 season, targeting the
LP.8.1 strain. The submission follows the recommendation by the EMA’s Emergency
Task Force (ETF) to update the COVID-19 vaccine composition for the coming
season to target the LP.8.1 strain. You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344 Related Report – ⬥︎ U.S. Clinical Trials Market - https://www.biospace.com/press-releases/u-s-clinical-trials-market-size-to-surpass-usd-75-39-billion-by-2034
⬥︎ Alzheimer’s Disease Diagnostics Market - https://www.biospace.com/press-releases/alzheimers-disease-diagnostics-market-size-to-reach-usd-25-53-billion-by-2034
⬥︎ U.S. DNA Manufacturing Market - https://www.biospace.com/press-releases/u-s-dna-manufacturing-market-size-to-reach-usd-14-75-billion-by-2034
⬥︎ U.S. Medical Device Contract Manufacturing Market - https://www.biospace.com/press-releases/u-s-medical-device-contract-manufacturing-market-size-to-hit-usd-62-42-billion-by-2034
⬥︎ Human DNA Vaccines Market - https://www.biospace.com/press-releases/human-dna-vaccines-market-size-expected-to-hit-usd-691-23-million-by-2034
⬥︎ U.S. Gastric Cancer Diagnostics Market - https://www.biospace.com/press-releases/u-s-gastric-cancer-diagnostics-market-size-share-and-growth-report-2034
⬥︎ U.S. Pharmaceutical CDMO Market - https://www.biospace.com/press-releases/u-s-pharmaceutical-cdmo-market-size-to-hit-usd-83-86-billion-by-2034
⬥︎ Leukemia Therapeutics Market - https://www.biospace.com/press-releases/leukemia-therapeutics-market-size-to-reach-usd-40-11-billion-by-2034
Segments Covered in the Report This report forecasts revenue growth at
country levels and provides an analysis of the latest industry trends in each
of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc.
has segmented the U.S. mRNA synthesis raw materials market By Type • Capping Agents • Nucleotides • Plasmid DNA • Enzymes o
Polymerase o
RNase Inhibitor o
DNase o
Others • Others By Application • Vaccine Production • Therapeutics Production • Others By End-use • Biopharmaceutical & Pharmaceutical
Companies • CROs & CMOs • Academic & Research Institutes Immediate Delivery Available | Buy This
Premium Research https://www.novaoneadvisor.com/report/checkout/8534
About-Us Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep, data-driven
insights that power innovation and transformation across industries. With a
sharp focus on the evolving landscape of life sciences, we specialize in
navigating the complexities of cell and gene therapy, drug development, and the
oncology market, enabling our clients to lead in some of the most revolutionary
and high-impact areas of healthcare. Our expertise spans the entire
biotech and pharmaceutical value chain, empowering startups, global
enterprises, investors, and research institutions that are pioneering the next
generation of therapies in regenerative medicine, oncology, and precision
medicine. Web: https://www.novaoneadvisor.com/ Contact Us USA: +1 804 420 9370 Email: sales@novaoneadvisor.com For Latest Update
Follow Us: LinkedIn