Policy
Jim O’Neill was deputy secretary at the Department of Health and Human Services and also acting director of the CDC after the abrupt ouster of Susan Monarez in August 2025.
FEATURED STORIES
Bristol Myers Squibb, GSK and Merck are contributing drug ingredients as part of their deals with the White House but are keeping many of the terms of their agreements private.
Some 200 rare disease therapies are at risk of losing eligibility for a pediatric priority review voucher, a recent analysis by the Rare Disease Company Coalition shows. That could mean $4 billion in missed revenue for already cash-strapped biotechs.
The FDA’s rare pediatric disease priority review voucher program missed reauthorization at the last minute in 2024; advocates have been fighting to get it back ever since.
Subscribe to BioPharm Executive
Market insights and trending stories for biopharma leaders, in your inbox every Wednesday
THE LATEST
The FDA granted Priority Review for omidubicel with a target action date of January 30, 2023. Omidubicel is a first-in-class, advanced NAM (nicotinamide)-enabled stem cell therapy.
The end of July is busy for the FDA, with Coherus, Sanofi, Acadia, Myovant and Pfizer having PDUFA dates filling the calendar. Here’s a look.
InMed updated its INM-755 for patients with epidermolysis bullosa, Seagen and Astellas announced positive topline results for Padcev with Merck’s Keytruda, and more.
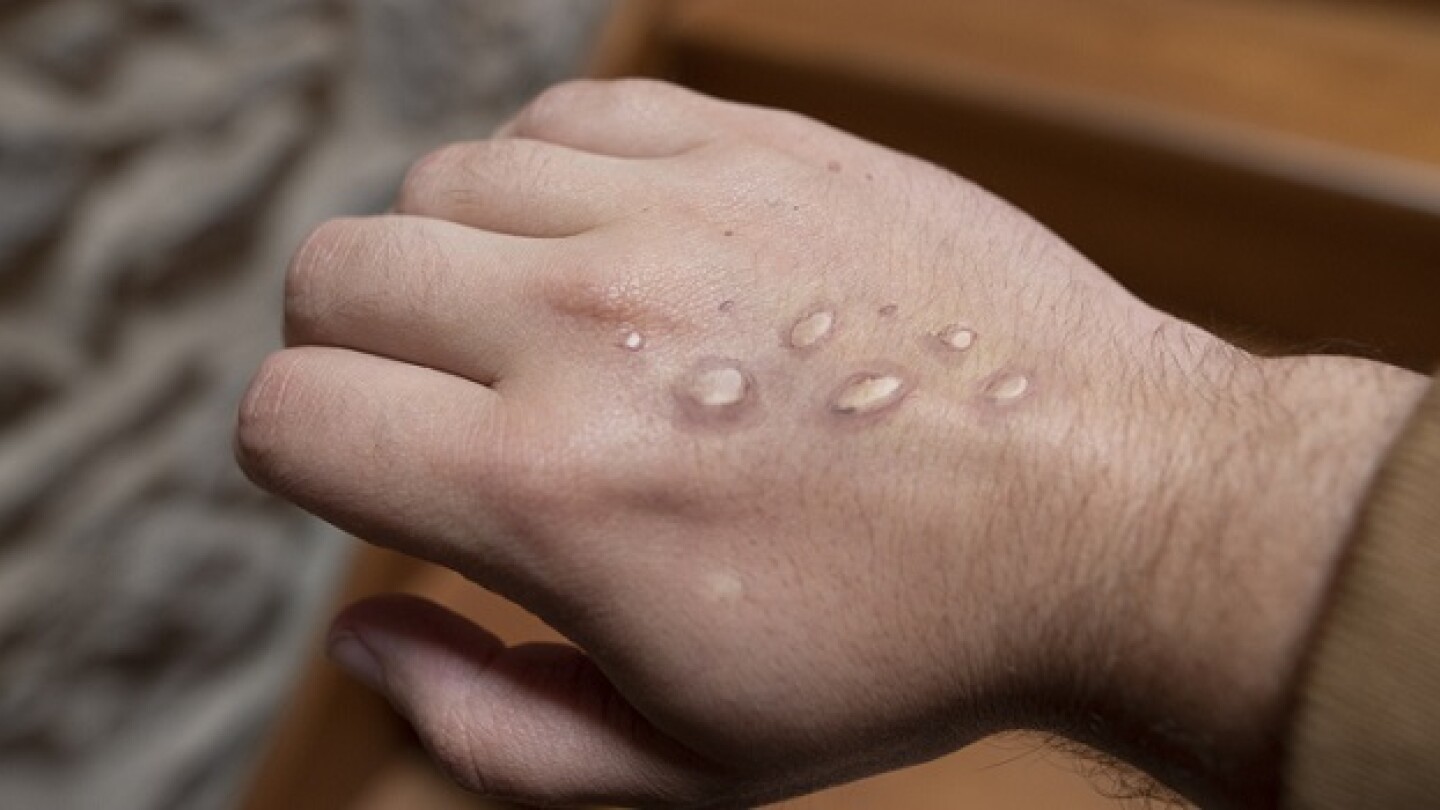
The measures will allow San Francisco and New York to coordinate efforts across different agencies and raise the public’s awareness to curb the spread of monkeypox.
The probe will be led by investigators experienced in determining whether or not entities have defrauded consumers, investors or government agencies.
Pfizer and BioNTech initiated a Phase II trial of an enhanced COVID-19 vaccine while fending off lawsuits against CureVac and Alnylam over their technology.
Teva expects to finalize and complete the documentation needed in the coming weeks, followed by a nationwide sign-on process for states, subdivisions and Native American tribes.
Martin Shkreli, commonly known as the “Pharma Bro,” announced Monday he has cofounded Druglike, a Web3 drug discovery software platform, despite a lifetime ban from the pharma industry.
WHO declared the monkeypox outbreak a “public health emergency of international concern [PHEIC].” As COVID-19 wanes, Tonix, SIGA, Emergent Bio and others are now targeting monkeypox.
The U.S. Attorney’s Office was busy indicting nine people for charges related to insider trading. Some charges were unrelated, but three were specific to the biopharma industry.