Policy
Jim O’Neill was deputy secretary at the Department of Health and Human Services and also acting director of the CDC after the abrupt ouster of Susan Monarez in August 2025.
FEATURED STORIES
Bristol Myers Squibb, GSK and Merck are contributing drug ingredients as part of their deals with the White House but are keeping many of the terms of their agreements private.
Some 200 rare disease therapies are at risk of losing eligibility for a pediatric priority review voucher, a recent analysis by the Rare Disease Company Coalition shows. That could mean $4 billion in missed revenue for already cash-strapped biotechs.
The FDA’s rare pediatric disease priority review voucher program missed reauthorization at the last minute in 2024; advocates have been fighting to get it back ever since.
Subscribe to BioPharm Executive
Market insights and trending stories for biopharma leaders, in your inbox every Wednesday
THE LATEST
CDC director Dr. Rochelle Walensky announced plans to launch a “reset” of the agency amid criticism over its handling of the COVID-19 pandemic and other public health threats.
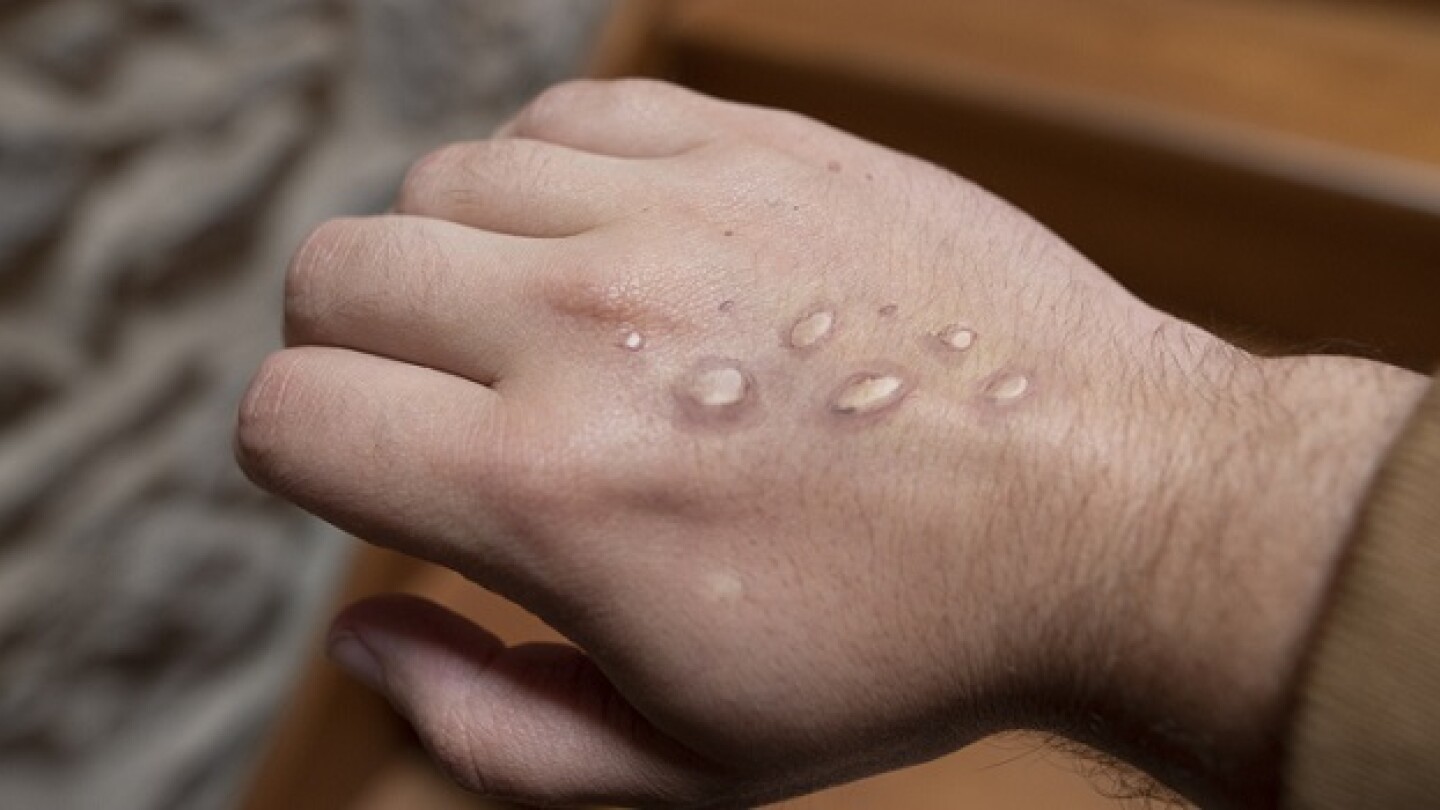
Cases of monkeypox are growing at an alarming rate and Bavarian Nordic, the company that owns one of the two approved vaccines, may no longer be able to keep up.
The FDA expects to foster innovation and competition among manufacturers, which would lower the prices of hearing aids without compromising their quality.
Blueprint Medicines’ Ayvakit is headed to the FDA for a new indication after the drug hit the mark in Part 2 of the PIONEER trial for non-advanced systemic mastocytosis.
The Bill & Melinda Gates Foundation inked a memorandum of understanding with South Korea’s foreign and health ministries to expand their partnerships revolving around public health.
Novavax seeks EUA for COVID-19 Booster, a 100-year-old TB vaccine may protect against the disease and public health officials struggle with fall planning.
The FDA has accepted the sNDA for AstraZeneca and Merck’s Lynparza and the sBLA for Genentech (Roche)'s for Polivy in diffuse large B-cell lymphoma.
As the biotech world awaits the announcement of Merck’s anticipated buyout of Seagen, an arbitrator came down on the side of Daiichi Sankyo in a patent battle against the Seattle-based biotech.
BrainStorm Cell Therapeutics said it will submit a Biologics License Application to the FDA for ALS hopeful NurOwn. Additionally, a correction has been made to analyses of the Phase III clinical trial.
Daiichi Sankyo and AstraZeneca report another first-in-class approval for Enhertu, Bayer snags an sNDA in metastatic hormone-sensitive prostate cancer and Merck faces contamination challenges with Januvia.