|
NEW HAVEN, Conn., Feb. 3, 2022 /PRNewswire/ --Biohaven Pharma Holding Company Ltd. (NYSE: BHVN) announced the publication of new findings from its long-term safety study showing reductions in migraine frequency in patients treated with rimegepant 75 mg for the acute treatment of migraine in the February issue of Cephalalgia Reports. Cephalalgia Reports is a medical-neurological journal in the field of headache research and is an official journal of the International Headache Society.
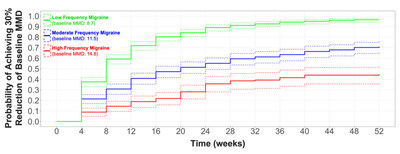
The published paper describes outcomes from patients treated with rimegepant 75 mg as needed (PRN) for the acute treatment of migraine, expressed as median time to ≥30% and ≥50% reduction in monthly migraine days (MMD). The 1044 patient study population was derived from the Open Label Safety Study BHV3000-201 (NCT03266588). This was a post-hoc analysis of adults with >6 MMD at baseline who self-administered rimegepant 75 mg orally PRN for acute treatment of migraine up to 52-weeks in an open-label safety study (BHV3000-201). This study is the first to utilize Kaplan–Meier and Cox Proportional Hazards methodology to describe longitudinal changes in migraine frequency.
Gil L'Italien Ph.D., Senior Vice President, GHEOR & Epidemiology, Biohaven, and lead author of the study, commented, "The Kaplan-Meier methodology provides for a novel assessment of migraine treatment efficacy and enables the ability to estimate adjusted hazard ratios for time to these outcomes (i.e., via Cox regression). These observations of migraine frequency reduction from repeated effective acute rimegepant treatment show reductions in migraine days after repeated acute therapy and are consistent with NURTEC ODT's known preventive effects."
Biohaven conducted the study in collaboration with health economic and outcomes research consultants led by Karissa Johnston, Ph.D., Scientific Director from Broadstreet HEOR, located in Vancouver, BC.
The published data shows that long-term acute treatment of migraine attacks with rimegepant 75 mg lowered monthly disease burden by reducing the number of migraine days, see Figure 1.
Clinically significant reduction in MMD frequency was observed over time regardless of baseline migraine frequency, including low-frequency (baseline MMD 8.7), moderate frequency (baseline MMD 11.5) and high-frequency (baseline MMD 14.8) cohorts. Adjusted (age and gender) hazard ratios for time to >30% and >50% in baseline MMD reductions were greatest for the lowest frequency cohort 5.47 (95% CI: 4.27, 7.00) for ≥30% MMD reduction and 5.74 (95% CI: 4.22, 7.81) for ≥50% MMD reduction, reflective of a faster median time to ≥30% and ≥50% MMD reduction for this cohort.
Dr. Johnston commented, "The study results confirm that continued use of rimegepant as an acute treatment to help stop a migraine can also lessen the number of migraine attacks a person has over time. This is a significant added benefit that healthcare providers and patients should consider when determining the right acute treatment plan."
NURTEC® ODT (rimegepant) was approved by the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine in February 2020 and for the preventive treatment of episodic migraine in May 2021.
About NURTEC ODT
NURTEC ODT (rimegepant) is the first and only calcitonin gene-related peptide (CGRP) receptor antagonist available in a quick-dissolve ODT formulation that is approved by the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine with or without aura and the preventive treatment of episodic migraine in adults. The activity of the neuropeptide CGRP is thought to play a causal role in migraine pathophysiology. NURTEC ODT is a CGRP receptor antagonist that works by reversibly blocking CGRP receptors, thereby inhibiting the biologic activity of the CGRP neuropeptide. The recommended dose of NURTEC ODT is 75 mg, taken as needed, up to once daily to treat or every other day to help prevent migraine attacks. For more information about NURTEC ODT, visit nurtec.com. The most common adverse reaction was nausea and abdominal pain/indigestion. Avoid concomitant administration of NURTEC ODT with strong inhibitors of CYP3A4, strong or moderate inducers of CYP3A or inhibitors of P-gp or BCRP. Avoid another dose of NURTEC ODT within 48 hours when it is administered with moderate inhibitors of CYP3A4. Please click here for full NURTEC ODT Prescribing Information and Patient Information.
About Migraine
Nearly 40 million people in the U.S. suffer from migraine and the World Health Organization classifies migraine as one of the 10 most disabling medical illnesses. Migraine is characterized by debilitating attacks lasting four to 72 hours with multiple symptoms, including pulsating headaches of moderate to severe pain intensity that can be associated with nausea or vomiting, and/or sensitivity to sound (phonophobia) and sensitivity to light (photophobia). There is a significant unmet need for new treatments as more than 90 percent of people with migraine are unable to work or function normally during an attack.
CGRP Receptor Antagonism
Small molecule CGRP receptor antagonists represent a novel class of drugs for the treatment of migraine. CGRP receptor antagonists work by reversibly blocking CGRP receptors, thereby inhibiting the biologic activity of the CGRP neuropeptide. For acute treatment, this unique mode of action potentially offers an alternative to other agents, particularly for patients who have contraindications to the use of triptans or who have a poor response to triptans or are intolerant to them. CGRP signal-blocking therapies have not been associated with medication overuse headache (MOH) or rebound headaches which limits the clinical utility of other acute treatments due to increases in migraine attacks that result from frequent use.
About Biohaven
Biohaven is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's Neuroinnovation™ portfolio includes FDA-approved NURTEC ODT (rimegepant) for the acute and preventive treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer's disease, and spinocerebellar ataxia; and MPO inhibition for amyotrophic lateral sclerosis. More information about Biohaven is available at www.biohavenpharma.com.
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements regarding the future development, timing and potential marketing approval and commercialization of NURTEC ODT (rimegepant), rimegepant or zavegepant. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K for the year ended December 31, 2020, filed with the Securities and Exchange Commission on March 1, 2021, and Biohaven's subsequent filings with the Securities and Exchange Commission. The forward-looking statements are made as of this date and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC. Neuroinnovation is a trademark of Biohaven Pharmaceutical Holding Company Ltd.
Biohaven Contact:
Vlad Coric, M.D.
Chief Executive Officer
Vlad.Coric@biohavenpharma.com
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502
 View original content to download multimedia:https://www.prnewswire.com/news-releases/nurtec-odt-when-taken-as-an-acute-treatment-for-migraine-reduces-monthly-migraine-days-expanded-data-from-long-term-treatment-published-in-cephalalgia-reports-301474512.html View original content to download multimedia:https://www.prnewswire.com/news-releases/nurtec-odt-when-taken-as-an-acute-treatment-for-migraine-reduces-monthly-migraine-days-expanded-data-from-long-term-treatment-published-in-cephalalgia-reports-301474512.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.
|






