Nanobiotix global development plan for first-in-class radioenhancer NBTXR3 across several solid tumor indications proceeds as planned with focus on priority pathways in head and neck cancer and immunotherapy

- Nanobiotix global development plan for first-in-class radioenhancer NBTXR3 across several solid tumor indications proceeds as planned with focus on priority pathways in head and neck cancer and immunotherapy
- Data from the expansion part of the phase I study in head and neck cancer showed:
- Target lesion objective response rate of 83.9%, confirming data reported earlier in 2020
- Target lesion complete response rate of 67.7%, which was an increase over previously reported data (60%) with additional follow up
- Dose escalation part of the phase I/II study in liver cancer completed successfully, with data showing:
- A target lesion objective response rate of 90.9% in evaluable hepatocellular carcinoma patients and a target lesion objective response rate of 71.4% in evaluable patients with liver metastasis
- A recommended phase II dose at 42% of tumor volume
- Preparation for pivotal phase III global registration trial in head and neck cancer is ongoing and the study is expected to launch after the financing to fund the trial is secured
- First clinical results from phase I trial evaluating NBTXR3 activated by radiation therapy in combination with pembrolizumab or nivolumab to be presented by the end of 2020
“While 2020 has been a challenging year for our patients, our industry, and the world, we have remained steadfast in our efforts to develop NBTXR3 with deliberate speed. We thank our team members, partners, and shareholders for the support that has allowed Nanobiotix to continue achieving necessary milestones for the product as planned. We are proud that we have endured in our collective commitment to advancing innovation with the potential to improve therapeutic outcomes for patients.” – Laurent Levy, CEO of Nanobiotix
PARIS & CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201026005205/en/
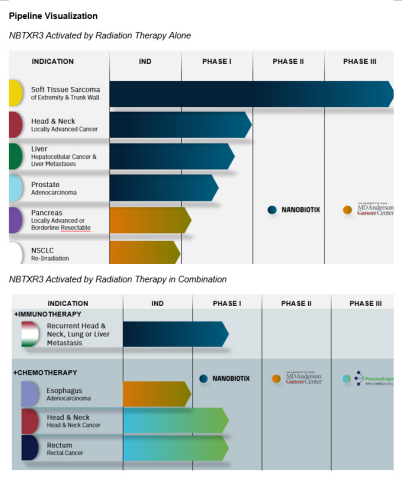
Pipeline Visualization (Photo: Business Wire)
NANOBIOTIX (Euronext : NANO – ISIN : FR0011341205 – the ‘‘Company’’) today announced updates to the Company’s global development plan for first-in-class radioenhancer NBTXR3 at the 2020 American Society for Radiation Oncology (ASTRO) Annual Meeting.
While the COVID-19 crisis and subsequent measures to curtail the spread of the virus have created challenges in clinical trial recruitment, a sharpened focus on the Company’s priority pathways in head and neck cancer and immunotherapy has led to the continued achievement of necessary milestones for the development of lead product candidate NBTXR3. Moreover, diligent efforts in collaboration with The University of Texas MD Anderson Cancer Center (MD Anderson) in the United States (US) has helped to advance expansion of the development plan across new indications.
Priority Development in Head and Neck Cancer and Immunotherapy
Pathway to Global Registration in Head and Neck Cancer On Track
Nanobiotix has presented an update on the expansion part of the Company’s phase I study evaluating NBTXR3 activated by radiation therapy for patients with head and neck cancer (Study 102) at ASTRO 2020. In 31 evaluable patients, positive results confirmed the target lesion objective response rate presented earlier this year at 83.9% and showed an increase in target lesion complete response rate at 67.7%. The target lesion complete response rate is an increase over previously reported data (60%) with additional follow up (7.8-month median follow up vs. 5 months). Additionally, data showed an overall objective response rate of 83.9% and an overall complete response rate of 48.4%, which were also increases over previously reported data (83% and 43% respectively).
The trial has recruited 44 patients in total (31 evaluable to date), and will remain ongoing until reaching 44 evaluable patients. The next update on the study is expected in the second quarter of 2021.
Preparation for the Company’s pivotal phase III trial evaluating NBTXR3 activated by radiation therapy for patients with head and neck cancer (NANORAY-312) is ongoing as planned. The trial is expected to commence after the financing to fund the trial is secured.
As previously announced, NBTXR3 activated by radiation therapy for the population in NANORAY-312 received Fast Track designation from the US Food and Drug Administration (FDA) in February 2020. Fast Track is a process designed to facilitate the development and accelerate the review of drugs for serious conditions and that have the potential to address unmet medical needs. The purpose is to expedite the availability of new treatment options for patients.
First Clinical Data in Immunotherapy to be Presented in Coming Weeks
Nanobiotix will present the first clinical results from a phase I study evaluating NBTXR3 activated by radiation therapy in combination with pembrolizumab or nivolumab for patients with head and neck cancer, lung metastasis and/or liver metastasis (Study 1100) by the end of 2020.
Nine patients have been injected in the trial thus far and recruitment remains ongoing. After the release of first results, the next step for the trial will be establishment of the recommended phase II dose (RP2D) by mid year 2021 for the head and neck cancer cohort and the lung metastasis cohort, and in the second half of 2021 for the liver metastasis cohort.
Early-Stage Development Across Other Solid Tumor Indications and with Collaborators
Nanobiotix Trials in Additional Solid Tumor Indications
The dose escalation part of the Company’s phase I/II trial evaluating NBTXR3 activated by radiation therapy for the treatment of patients with hepatocellular carcinoma (HCC) or liver metastasis (Study 103) has completed successfully and final data has been presented at ASTRO 2020. Data showed that the product candidate continues to be safe and well tolerated with no dose-limiting toxicities (DLTs). Early efficacy data showed a target lesion objective response rate of 90.9% in evaluable patients with hepatocellular carcinoma (HCC) and a target lesion objective response rate of 71.4% in evaluable patients with liver metastasis. Further development in this indication will proceed after the launch of the Company’s phase III head and neck cancer trial.
The Company’s phase I trial evaluating NBTXR3 activated by radiation therapy for the treatment of patients with prostate cancer (Study 104) has administered NBTXR3 to five patients, with no serious adverse events and no DLTs reported. At present the Company has paused development in this indication to focus resources on the head and neck cancer and immunotherapy pathways.
In soft tissue sarcoma, the final patient follow up visit has been completed in the phase III Act.In.Sarc study. The planned post-registrational trial is expected to launch in 2021.
Trials with Collaborators
The Company’s clinical collaboration with MD Anderson continues to move forward with the launch of a phase I trial evaluating NBTXR3 activated by radiation therapy for patients with pancreatic cancer. FDA ‘Safe to Proceed’ notifications have been received for four additional trials in the collaboration and are pending activation.
***
About NBTXR3
NBTXR3 is a first-in-class radioenhancer composed of functionalized hafnium oxide nanoparticles that is administered via one-time intra-tumoral injection and activated by radiation therapy. The physical and universal mode of action (MoA) of NBTXR3 is designed to trigger cellular destruction death and adaptive immune response.
NBTXR3 is being evaluated in locally advanced head and neck squamous cell carcinoma (HNSCC) of the oral cavity or oropharynx in elderly patients unable to receive chemotherapy or cetuximab with limited therapeutic options. Promising results have been observed in the phase I trial regarding local control. In the United States, the Company has started the regulatory process to commence a phase III clinical trial in locally advanced head and neck cancers. In February 2020, the United States Food and Drug Administration granted the regulatory Fast Track designation for the investigation of NBTXR3 activated by radiation therapy, with or without cetuximab, for the treatment of patients with locally advanced head and neck squamous cell cancer who are not eligible for platinum-based chemotherapy.
Nanobiotix is also running an Immuno-Oncology development program. The Company has launched a Phase I clinical trial of NBTXR3 activated by radiotherapy in combination with anti-PD-1 checkpoint inhibitors in locoregional recurrent (LRR) or recurrent and metastatic (R/M) HNSCC amenable to re-irradiation of the HN and lung or liver metastases (mets) from any primary cancer eligible for anti-PD-1 therapy.
Other ongoing NBTXR3 trials are treating patients with hepatocellular carcinoma (HCC) or liver metastases, locally advanced or unresectable rectal cancer in combination with chemotherapy, head and neck cancer in combination with concurrent chemotherapy, and pancreatic cancer. The Company is also engaged in a broad, comprehensive clinical research collaboration with The University of Texas MD Anderson Cancer Center to further expand the NBTXR3 development program.
About NANOBIOTIX: www.nanobiotix.com
Incorporated in 2003, Nanobiotix is a leading, clinical-stage nanomedicine company pioneering new approaches to significantly change patient outcomes by bringing nanophysics to the heart of the cell.
The Nanobiotix philosophy is rooted in designing pioneering, physical-based approaches to bring highly effective and generalized solutions to address unmet medical needs and challenges.
Nanobiotix’s first-in-class, proprietary lead technology, NBTXR3, aims to expand radiotherapy benefits for millions of cancer patients. Nanobiotix’s Immuno-Oncology program has the potential to bring a new dimension to cancer immunotherapies.
Nanobiotix is listed on the regulated market of Euronext in Paris (Euronext: NANO / ISIN: FR0011341205; Bloomberg: NANO: FP). The Company’s headquarters are in Paris, France, with a US affiliate in Cambridge, MA, and European affiliates in France, Spain and Germany.
Disclaimer
This press release contains certain forward-looking statements concerning Nanobiotix and its business, including its prospects and product candidate development. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forward-looking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the universal registration document of Nanobiotix registered with the French Financial Markets Authority (Autorité des Marchés Financiers) under number R.20-010 on May 12, 2020 (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201026005205/en/
Nanobiotix
Communications Department
Brandon Owens
VP, Communications
+1 (617) 852-4835
contact@nanobiotix.com
Investor Relations Department
Ricky Bhajun
Senior Manager, Investor Relations
+33 (0)1 79 97 29 99
investors@nanobiotix.com
Media Relations
France – Ulysse Communication
Pierre-Louis Germain
+ 33 (0)6 64 79 97 51
plgermain@ulysse-communication.com
Source: NANOBIOTIX
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20201026005205/en







