Fluidx Medical announces the completion of a multi-center clinical trial examining the broad application of their new liquid embolic agent, GPX®.
Completion of Trial is a Sign of Things to Come for Novel Embolic Agent
SALT LAKE CITY, Oct. 6, 2022 /PRNewswire/ -- Fluidx Medical announces the completion of a multi-center clinical trial examining the broad application of their new liquid embolic agent, GPX®. Embolization is a procedure in which arterial or venous blood supply is intentionally blocked.
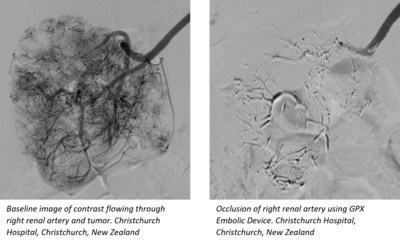
"In the study, GPX was successfully used to treat a range of hypervascular tumors as well as other arterial and venous applications", stated Martin Krauss, M.D., Head of Interventional Radiology at Christchurch Hospital. "The embolic flowed nicely, providing excellent control and beautiful filling of distal branches."
With the positive conclusion of this trial, the Fluidx embolic platform has a promising future. "The completion of this trial gives GPX a lot of momentum," said Russ Bjorklund, Vice President of Strategy and Marketing. "Physician feedback surpassed expectations, and the results of this trial give us a great deal of confidence in GPX as a platform technology with demonstrated value in peripheral vascular and interventional oncology, and opportunities for expansion to neurovascular applications."
A common concern with liquid embolics is that the delivery microcatheter tip may become entrapped in solidifying embolic following deployment. But the unique GPX material does not entrap existing delivery microcatheters, and no entrapment was observed during the study. Because clinicians weren't concerned with GPX-caused microcatheter entrapment, they were able to take their time during material delivery to ensure complete occlusion of the targeted region.
GPX technology is a low viscosity, aqueous-based solution that solidifies into a durable embolus upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation.* The platform is expected to bring simple preparation and controllable material delivery to several embolic applications. The device is packaged in a ready-to-use syringe, can be prepped tableside by the clinician in about 30 seconds, and may be delivered through standard microcatheters (no complex mixing systems or special delivery catheters are necessary).
Fluidx Medical Technology is a Salt Lake City, Utah based company focused on developing the GPX Embolic Device and other embolic technologies with applications across peripheral vascular, interventional oncology, and neurovascular.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time.
*Data on file. Fluidx Medical.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/multi-center-liquid-embolic-study-concludes-301641958.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/multi-center-liquid-embolic-study-concludes-301641958.html
SOURCE Fluidx Medical Technology