In a new clinical trial looking at the efficacy of a third Moderna vaccine dose for coronavirus disease 2019 (COVID-19) in people living with an organ (liver or kidney) transplant, The Feinstein Institutes for Medical Research administered the extra vaccine to the first set of patients in the United States on September 20.
First in the nation, researchers dose transplant patients with third vaccine dose in new clinical trial
MANHASSET, N.Y.--(BUSINESS WIRE)-- In a new clinical trial looking at the efficacy of a third Moderna vaccine dose for coronavirus disease 2019 (COVID-19) in people living with an organ (liver or kidney) transplant, The Feinstein Institute – the science arm of Northwell Health – administered the extra vaccine to the first set of patients in the United States on September 20. The multi-centered, nationwide clinical trial is sponsored by the vaccine-maker Moderna.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210921005840/en/
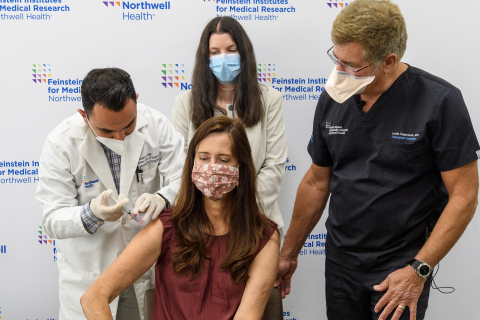
Darla Smyth (center) an organ transplant recipient receives her third dose of Moderna COVID-19 vaccine as part of a new clinical trial at the Feinstein Institutes. (Credit: Feinstein Institutes)
People who have received an organ transplant are at a high-risk for contracting COVID-19 and becoming severely sick. After receiving a transplant organ, immunosuppressive drugs may interfere with the recipient’s ability to build a strong immune defense against the virus from the first doses of the virus. This trial will dose patients with an extra shot and monitor their immune antibody response 28 days from the third shot.
“Organ transplant recipients may not have as strong of an immune response to the COVID-19 vaccines as the general population does, leaving them vulnerable to the virus,” said Lewis Teperman, MD, director of transplant services for Northwell Health and principal investigator on the trial. “We are eager to provide patients more vaccine to help protect them, and to gain much-needed scientific evidence to help doctors best treat their patients.”
The first trial participant to receive the shot – and first in the nation – was Darla Smyth, PhD, a high school English teacher from Hewett-Woodmere. After being diagnosed with Primary Biliary Cirrhosis more than 28 years ago and undergoing a liver transplant, Dr. Smyth was eager to roll up her sleeve. “When you weigh the options – either get sick or get vaccinated again, my choice was clear. I am hoping this third shot will help give me some lasting protection from this virus.”
People who have received a solid organ transplant within the last six months may qualify for the trial. Moderna is hoping 240 participants (including healthy volunteers) will be enrolled in the trial nationwide. The Feinstein Institutes is one of the seven sites leading the trial and the first to enroll.
“Since the start of the COVID-19 pandemic, the Feinstein Institutes focused on conducting clinical trials in an effort to find safe and effective treatments,” said Christina Brennan, MD, vice president of clinical research at the Feinstein Institutes. “Now, with the support of Moderna, we hope this vaccine clinical trial will give insight into the best way to protect a high-risk population.”
In March 2020, the Feinstein Institutes announced its first set of clinical trials focused on studying the safety and efficacy of potential COVID-19 therapies. Since then, Feinstein Institutes initiated more than 17 clinical trials and programs and enrolled more than 1,800 patients. Through Feinstein’s COVID-19 Research Consortium (CRC), 500 clinicians, statisticians and scientists published more than 500 peer-reviewed manuscripts related to the virus in efforts to inform the public and research community.
“Clinical trials are essential to define effective methods to treat and prevent COVID-19,” said Kevin J. Tracey, MD, president and CEO of the Feinstein Institutes. “Dr. Teperman’s trial to study vaccination of organ transplant patients is timely and important.”
Most recently, the Feinstein Institutes began to enroll patients in another national clinical trial that is delivering extra shots of vaccines to patients with an autoimmune disease. Researchers will investigate whether antibody response to the vaccine is related to medications, disease and/or vaccine type.
More information about the organ transplant trial, including the locations of study sites, is available at ClinicalTrials.gov (NCT04860297).
About the Feinstein Institutes
The Feinstein Institutes for Medical Research is the research arm of Northwell Health, the largest health care provider and private employer in New York State. Home to 50 research labs, 3,000 clinical research studies and 5,000 researchers and staff, the Feinstein Institutes raises the standard of medical innovation through its five institutes of behavioral science, bioelectronic medicine, cancer, health system science, and molecular medicine. We make breakthroughs in genetics, oncology, brain research, mental health, autoimmunity, and are the global scientific leader in bioelectronic medicine – a new field of science that has the potential to revolutionize medicine. For more information about how we produce knowledge to cure disease, visit http://feinstein.northwell.edu and follow us on LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210921005840/en/
Source: Feinstein Institutes for Medical Research