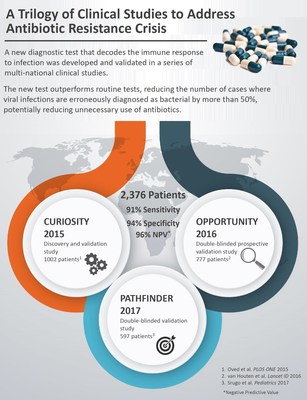
TIRAT CARMEL, Israel, September 18, 2017 PRNewswire/ -- MeMed today announced positive results from a large, external double-blinded PATHFINDER study that met its primary endpoint, independently confirming that MeMed's novel blood test, ImmunoXpert, accurately distinguishes between bacterial and viral infections in children. The results were published in the online version of Pediatrics, the official journal of the American Academy of Pediatrics. PATHFINDER completes a trilogy of clinical studies conducted over the past seven years that include CURIOSITY (published in PLOS One 2015) and OPPORTUNITY (published in Lancet Infectious Diseases 2016), which collectively enrolled 2,376 patients. The test aims to support clinicians in one of the most routine yet challenging clinical dilemmas today - determining whether an infection is bacterial or viral in order to decide whether to treat or not to treat with antibiotics.
"It takes years of work and thousands of patients to reliably establish the diagnostic performance of a new test, particularly one that can help revolutionize patient care," said Eran Eden, PhD, MeMed CEO. "The findings of these three clinical studies enrolling over 2,000 patients, together with additional data emerging from early access medical centers that have already used the CE-IVD certified test to support management of over 10,000 patients, have generated exceptional high quality clinical evidence. These results will drive market adoption and further solidify MeMed as a world leader in immune-based diagnostics."
PATHFINDER is an international, multi-center, external double-blinded, clinical study that enrolled 597 pediatric patients with suspected acute infection including fever without identifiable source, upper and lower respiratory tract infections, urine tract infections, and non-infectious controls. The study, which took place in Switzerland and Israel, was led by Prof. Alain Gervaix, MD, Chief Medical Officer of the Pediatric Emergency Department at Geneva University Hospital and Prof. Isaac Srugo, MD, Chief of the Pediatric Department and Director of Microbiology Laboratory at Bnai-Zion Medical Center in Israel.
An independent Commentary by Dr. Kimberlin, President of the Pediatric Infectious Diseases Society (2015-2017), noted: "No single test has proven adept at accurately distinguishing the child with a life-threatening infection from the child who will get better on his or her own. Into this terrain, Srugo et al increase the number of tools in our toolbox and potentially move us substantially closer to that Holy Grail of accurately determining which child truly is at risk for having a serious bacterial infection", (Kimberlin and Poole, Pediatrics 2017).
The study demonstrated that the blood test accurately distinguished between bacterial and viral patients with 94% sensitivity and 90% specificity, and a negative predictive value of 98%. The test outperformed routine laboratory parameters used to manage patients with infections, including white blood count, C-reactive protein and procalcitonin. Compared to routine care the test significantly reduced both false negatives, potentially reducing missed bacterial infections, and false positives, potentially reducing antibiotic overuse.
"One unique aspect of PATHFINDER study was the double-blind design," commented Prof. Gervaix. "This type of strict study design is rarely applied in our field, and increases confidence in the results. "Prof. Srugo added: "Importantly, we found that the test may be applicable to harder-to-diagnose cases, as it assigned a clear diagnosis to the majority of cases where there was no agreement on the underlying cause of infection among three independent senior physicians examining the patient medical records. This is a critical capability as it allows clinicians to use the test confidently even when they are unsure what is causing the infection."
"Generating clinical evidence of the highest quality has always been our strategy toward better patient care," said Kfir Oved, PhD, MeMed CTO. "However, this is not enough by itself. To allow access to patients around the globe across different clinical settings, the test must be easy to use and provide results within minutes. We are progressing with the development of our point-of-care platform, with support from the US Department of Defense, to enable the use of the test where and when it is most needed."
About MeMed
MeMed is dedicated to improving patient care through research, development and commercialization of solutions that decode the immune system's distinct responses to different health and disease states. The company focuses on providing rapid, accurate and actionable tests for acute infectious diseases and inflammatory disorders to be used in the hospital and community. Its first-generation test, ImmunoXpert, accurately detects whether a patient has a bacterial or viral infection, with the aim of empowering physicians to make more informed antibiotic treatment decisions. ImmunoXpert is cleared for clinical use in the EU (CE-IVD certified), Switzerland and Israel. It is currently in pilot distribution in these territories with a broader commercial roll-out underway. MeMed's second-generation test will provide rapid (within minutes) results at the point-of-care. The company is partnering with international stakeholders from industry and government to facilitate global availability of its tests, and has plans underway to initiate clinical studies in the U.S. in 2017. For additional information, please visit http://www.me-med.com.
Company Contact:
Asi Cohen-Dotan, PhD, MeMed
Phone: +972-4-8500302
asi.cohen@me-med.com
Media Contact:
Kirsten Thomas, The Ruth Group
Phone: +1-508-280-6592
kthomas@theruthgroup.com

View original content with multimedia:http://www.prnewswire.com/news-releases/memed-completes-trilogy-of-clinical-studies-with-more-than-2000-patients-validating-first-ever-blood-test-to-accurately-distinguish-between-bacterial-and-viral-infections-300521005.html
SOURCE MeMed




