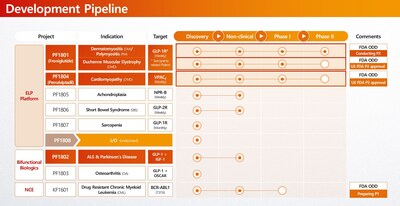
- Focusing on global out-licensing of Phase 2 assets PF1801 and PF1804.
- Company to engage with global big pharma in San Francisco to accelerate U.S. clinical entry.
SEOUL, South Korea, Jan. 1, 2026 /PRNewswire/ -- ImmunoForge Co., Ltd. (Co-CEOs Sung-Min Ahn and Kiho Chang), a clinical-stage biopharmaceutical company specializing in rare diseases, today announced that its leadership team will be in San Francisco during the 43rd Annual J.P. Morgan Healthcare Conference (January 12–15, 2026) to conduct strategic 1-on-1 meetings with global pharmaceutical companies and investors.
Instead of a formal stage presentation, ImmunoForge is focusing on high-level private partnering sessions to discuss the clinical progression and licensing of its key pipelines. The company's primary objective is to secure strategic partners capable of spearheading U.S. clinical trials for its Phase 2 assets.
Key Highlights for Partnering:
- Monthly long-acting ELP (Elastin-Like Polypeptide) platform: Peptide conjugation and Protein fusion possible
- BBB penetrating ELP (Elastin-Like Polypeptide) platform: BBB shuttle platform fusing ELP to make monthly CNS drugs
- PF1801 (Polymyositis/Dermatomyositis, DMD & Sarcopenia): Currently in a Phase 2 study in South Korea. With U.S. FDA Phase 2 IND clearance already secured for both indications, the asset is primed for an immediate U.S. clinical launch by a global partner.
- PF1804 (DMD Cardiomyopathy): This promising asset also received FDA Phase 2 IND clearance, standing ready for clinical execution.
- PF1807 (Sarcopenia): A monthly sarcopenia therapy based on use patent for the prevention and treatment of myositis and sarcopenia.
- KF1601 (CML): While domestic rights have been successfully licensed out ahead of a Phase 1 launch in Korea next year, global licensing rights (excluding Korea) are open for discussion, offering a validated opportunity for international partners.
"Our goal for JPM 2026 week is to meet with potential partners who can leverage our FDA-cleared INDs to rapidly bring these therapies to the U.S. market," said a representative from ImmunoForge. "As a lean biotech, we have focused on de-risking our assets through regulatory clearances; we are now looking for the right global collaborators to take these programs through the next phase of clinical development and to the commercial markets. In addition to licensing deals with global pharmaceutical companies, we are also actively willing to pursue NewCo models led by investors. In line with this strategy, we intend to expand our development capabilities by introducing our diverse pipeline to global companies, including our established long-acting ELP platform and the newly developed BBB-penetrating ELP platform technology."
Schedule for Meeting
ImmunoForge is currently accepting meeting requests for the week of JPM 2026. To schedule a session with our executive team in San Francisco, please contact:
Business Development Team
- Email: [jim.ballance@immunoforge.com]
- Website: [www.immunoforge.com]
About ImmunoForge
ImmunoForge is a clinical-stage, venture-backed biopharmaceutical company dedicated to developing innovative therapies for muscular and rare diseases. The company leverages its proprietary long-acting ELP (Elastin-Like Polypeptide) platform technology to extend the half-life of peptide-based drugs, enabling weekly or monthly dosing schedules.
The company's lead candidate, PF1801 (froniglutide), is currently in a Phase 2 clinical trial for Polymyositis (PM) and Dermatomyositis (DM) in South Korea. Recognizing its therapeutic potential, the U.S. FDA has granted Orphan Drug Designation (ODD) to PF1801 for PM, DM, and Duchenne Muscular Dystrophy (DMD). Additionally, PF1804 (pemziviptadil) has received U.S. FDA IND approval for a Phase 2 study in DMD-associated cardiomyopathy and is ready for clinical commencement with strategic partners.
ImmunoForge is also advancing a diverse early-stage pipeline, including PF1802 (CNS), PF1803 (Osteoarthritis), and KF1601 (Oncology). To accelerate the delivery of these transformative medicines to patients worldwide, ImmunoForge is actively seeking global co-development and licensing partnerships for both its pipeline assets and its ELP platform technology.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/immunoforge-to-host-strategic-partnering-meetings-during-jp-morgan-healthcare-conference-2026-302650146.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/immunoforge-to-host-strategic-partnering-meetings-during-jp-morgan-healthcare-conference-2026-302650146.html
SOURCE ImmunoForge