icotec Medical, Inc. a US-based subsidiary of Swiss icotec AG today announced that it will unveil its VADER®one pedicle screw system during the 34th annual meeting of the North American Spine Society in Chicago, September 25-28, 2019.
|
NEW YORK, Sept. 19, 2019 /PRNewswire/ -- icotec Medical, Inc. a US-based subsidiary of Swiss icotec AG today announced that it will unveil its VADER®one pedicle screw system during the 34th annual meeting of the North American Spine Society in Chicago, September 25-28, 2019.
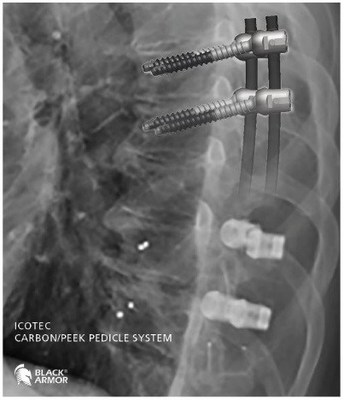
VADER®one, made from icotec's unique BlackArmor® Carbon/PEEK material, is cleared by the FDA for restoration of the spinal column in advanced stage tumor patients. By avoiding imaging artifacts, the radiolucent BlackArmor® material specifically enhances the outcome of x-ray, CT and MRI modalities. Patients can now be considered for a larger variety of treatment options with improved therapy planning, dose delivery and follow-up evaluation. A panel of leading surgeons selected the VADER®one pedicle screw system for the 2019 Spine Technology Award. The winning entries will be announced at NASS 2019. "We are extremely proud that VADER®one was selected as one of the most innovative, enduring and practical products in 2019," says Roger Stadler, CEO of icotec AG, Switzerland.
With icotec's system, surgeons don't have to change their standard surgical technique as VADER®one allows for minimally invasive or open surgical spine approaches. During NASS 2019 experienced US tumor surgeons will be present at icotec's booth to share their expertise and experience peer-to-peer with their colleagues each day during the meeting breaks. Hands-on demonstrations to the instrument and implant sets as well as insights into the unique BlackArmor® technology will be presented. The icotec team is looking forward to meeting you at booth #3727 during NASS 2019 in Chicago.
icotec Medical, Inc. is a subsidiary of icotec AG, a family-owned SME in Altstaetten, Switzerland established in 1999. icotec designs and manufactures nonmetallic spinal implants made from BlackArmor® (Carbon/PEEK). icotec's proprietary BlackArmor® material consists of continuous carbon fibers combined with PEEK (polyetheretherketone), and is produced using icotec's injection molding CFM (Composite Flow Molding) manufacturing technology. For more information, visit our website www.icotec-medical.com or contact us at media@icotec-medical.com
SOURCE icotec Medical, Inc. |