Hancock Jaffe Laboratories, Inc. (NASDAQ:HJLI)(NASDAQ:HJLIW), a developer of medical devices that restore cardiac and vascular health, announced today that the eleventh and final VenoValve patient in HJLIs first-in-human, clinical study has successfully reached the one-year milestone. Patient 11&39s chronic venous insufficiency
Final Patient in First-in-Human Study Shows Significant Improvement at One Year
IRVINE, CA / ACCESSWIRE / December 14, 2020 / Hancock Jaffe Laboratories, Inc. (NASDAQ:HJLI)(NASDAQ:HJLIW), a developer of medical devices that restore cardiac and vascular health, announced today that the eleventh and final VenoValve patient in HJLI's first-in-human, clinical study has successfully reached the one-year milestone. Patient 11's chronic venous insufficiency ("CVI") has dramatically improved when compared to pre-surgery levels, with reflux (the backwards flow of blood) improving 70%, disease manifestations, as measured by a venous clinical severity scores ("VCSS") improving 69%, and pain, as measured on a visual analog scale ("VAS"), improving 100%.
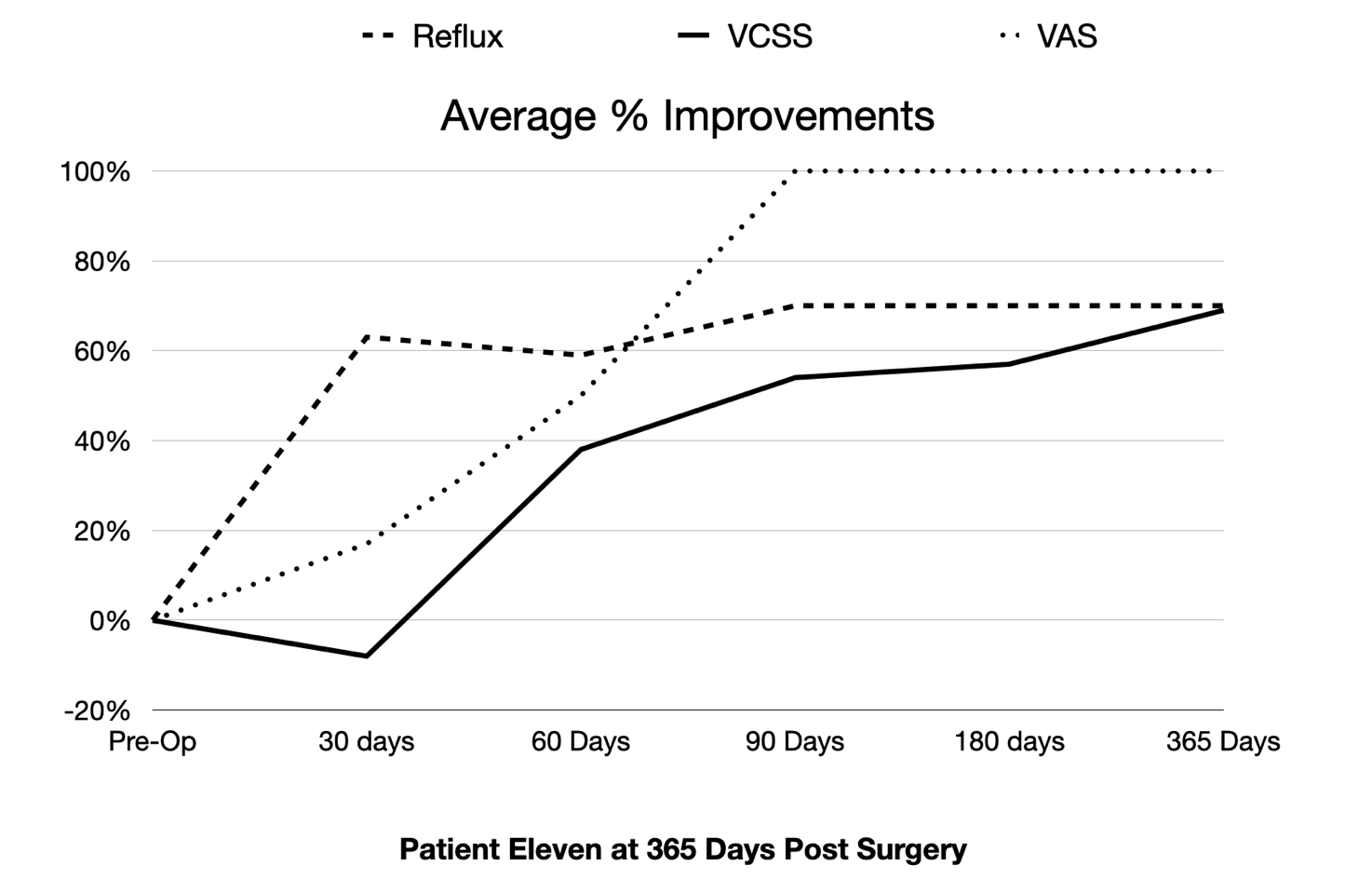
The patient's pre-surgery levels for reflux, VCSS, and VAS were 2.7, 13, and 6, respectively. At one-year post surgery, those levels were 0.8, 4, and 0, respectively. The improvement in VCSS score means that the patient went from having severe CVI to a very mild form of the disease. A VAS score of 0 means that the patient is completely pain-free. All eleven VenoValve patients have now completed the one-year first-in-human trial. HJLI expects to release aggregate data for all eleven patients in the coming days.
Dr. Marc H. Glickman, Hancock Jaffe's Senior Vice President and Chief Medical Officer stated, "The results for Patient 11 are impressive. We are gaining a lot of attention within the vascular community based upon our recent JVS VL ( Journal of Vascular Surgery Venous and Lymphatic Disorders ) article and our momentum is building. We are looking forward to sharing our data with the FDA as part of the IDE process and have begun preparations to file the IDE application and for the U.S. pivotal trial."
A paper containing preliminary results from the VenoValve first-in-human trial was recently published in the Journal of Vascular Surgery Venous and Lymphatic Disorders, the premier peer reviewed journal for vascular surgeons and other health care professionals engaged in the treatment of vascular disease. A copy of the paper can be accessed at https://doi.org/10.1016/j.jvsv.2020.10.017.
CVI occurs when the valves in the veins of the leg are injured or destroyed, causing blood to flow backwards, which is known as reflux. Reflux results in increased venous pressure (venous hypertension), damage to the veins, and results in the pooling of blood in the lower leg. Deep venous CVI is a serious condition, often resulting in debilitating pain, swelling, and open sores (venous ulcers) on the lower leg.
Next steps for the VenoValve include a Pre-IDE meeting with the U.S. Food and Drug Administration ("FDA"), currently scheduled for January 11, 2021, the completion of a series of functional tests and a GLP safety study mandated by the FDA, and the filing of an IDE application with the FDA, seeking approval to begin the U.S. pivotal trial, which HJLI expects to file in the first quarter of 2021. An investigational device exemption or IDE from the FDA is required for a medical device company to proceed with a pivotal trial in the U.S. for a class III medical device.
Approximately 2.4 million people in the U.S. suffer from CVI due to reflux in the deep venous system. Estimates indicate that direct medical costs from CVI in the U.S. exceed $38 Billion a year. There are currently no FDA approved devices, or effective treatments for deep venous CVI.
About Hancock Jaffe Laboratories, Inc.
Hancock Jaffe Laboratories (NASDAQ:HJLI) specializes in developing and manufacturing bioprosthetic (tissue based) medical devices to establish improved standards of care for treating cardiac and vascular diseases. Hancock Jaffe currently has two lead product candidates: the VenoValve®, a porcine based valve which is intended to be surgically implanted in the deep venous system of the leg to treat reflux associated with Chronic Venous Insufficiency; and the CoreoGraft®, a bovine tissue based off the shelf conduit intended to be used for coronary artery bypass surgery. Hancock Jaffe has a 20-year history of developing and producing FDA approved medical devices that sustain or support life. The current management team at Hancock Jaffe has been associated with over 50 FDA or CE marked medical devices. For more information, please visit HancockJaffe.com.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of Hancock Jaffe Laboratories, Inc. (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results (including, without limitation, the performance of the new board members described herein) may differ significantly from those set forth or implied in the forward-looking statements. These forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
SOURCE: Hancock Jaffe Laboratories, Inc.
View source version on accesswire.com:
https://www.accesswire.com/620702/Hancock-Jaffe-Announces-One-Year-Follow-up-Data-on-Eleventh-VenoValve-Patient




