The global Addison’s disease therapeutics market is expected to post a CAGR of close to 4% during the period 2019-2023, according to the latest market research report by Technavio.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190513005308/en/
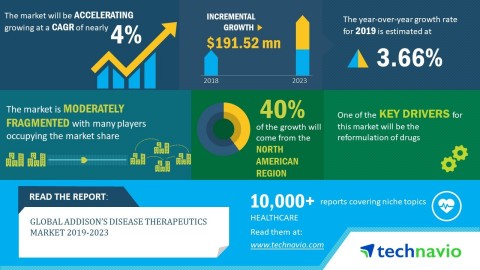
Technavio has published a new market research report on the global Addison's diseases therapeutics market from 2019-2023. (Graphic: Business Wire)
A key factor driving the growth of the global Addison's disease therapeutics market is the reformulation of drugs. The modified-release, sustained-release, extended-release, controlled-release, and prolonged-release versions of existing oral corticosteroid are gaining immense traction. This is mainly due to the pharmacodynamic and pharmacokinetic advantages of these versions over the conventional dosage forms. For instance, the prolonged action dosage forms maximize the bioavailability of the drug with a minimum dose and increase the rate as well as the extent of drug absorption. These benefits have led to the emergence of the reformulated versions of corticosteroids, which helps in the adequate maintenance of corticosteroid levels in the patients with Addison’s disease.
As per Technavio, the expanding research into the development of regenerative therapy will have a positive impact on the market and contribute to its growth significantly over the forecast period. This global Addison’s disease therapeutics market 2019-2023 research report also analyzes other significant trends and market drivers that will affect market growth over 2019-2023.
Global Addison’s disease therapeutics market: Expanding research into the development of regenerative therapy
Currently, the options available for the treatment of Addison’s disease involve the life-long use of exogenous corticosteroids. The long-term use of these drugs predisposes the patients to a range of harmful side effects such as buffalo hump, headache, muscle weakness, cataract, glaucoma, facial hair growth, and puffiness of the face. Thus, researchers are focusing on the development of regenerative therapies for the treatment of Addison's disease to reduce the duration of therapy and overcome these side effects. The adoption of regenerative medicines will significantly increase due to their minimal side effects and curative nature. Various research institutions and companies are investing in R&D of these medicines for curing rare and orphan diseases including Addison's disease. Such expanding research on the development of regenerative therapy will positively impact market growth during the forecast period.
“Apart from the expanding research into the development of regenerative therapies, factors such as the availability of clinical guidelines, redefined pathophysiology, and the adverse effects associated with available drugs are expected to have a significant impact on the growth of the Addison’s disease therapeutics market during the forecast period,” says a senior research analyst at Technavio.
Global Addison’s disease therapeutics market: Segmentation analysis
This market research report segments the global Addison's disease therapeutics market by product (oral drugs, and parenteral drugs) and geographic regions (North America, Europe, Asia, and ROW).
The North American region led the market in 2018, followed by Europe, Asia, and ROW respectively. The market growth in North American can be attributed to the presence of favorable reimbursement schemes, the increasing awareness about the diagnosis and effective management of Addison’s disease, and the market entry of orphan drugs with expanded exclusivity rights.
Looking for more information on this market? Request a free sample report
Technavio’s sample reports are free of charge and contain multiple sections of the report such as the market size and forecast, drivers, challenges, trends, and more.
Some of the key topics covered in the report include:
Market Landscape
- Market ecosystem
- Market characteristics
- Market segmentation analysis
Market Sizing
- Market definition
- Market size and forecast
Five Forces Analysis
Market Segmentation
Geographical Segmentation
- Regional comparison
- Key leading countries
Market Drivers
Market Challenges
Market Trends
Vendor Landscape
- Vendors covered
- Vendor classification
- Market positioning of vendors
- Competitive scenario
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 10,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
If you are interested in more information, please contact our media team at media@technavio.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20190513005308/en/
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
www.technavio.com
Source: Technavio Research







