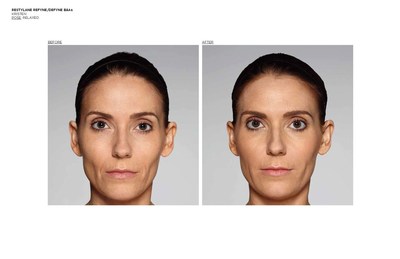
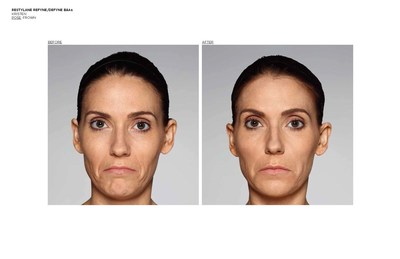
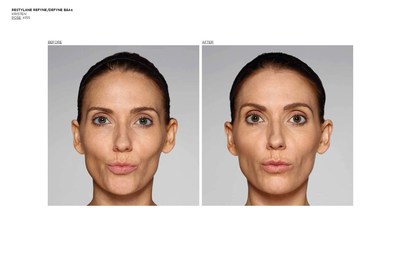
FORT WORTH, Texas, Dec. 12, 2016 /PRNewswire/ -- Galderma, a global leader focused on medical solutions in skin health, announced today it has received U.S. Food and Drug Administration (FDA) approval of two new products for the treatment of nasolabial folds (NLF) or "laugh lines," in patients over the age of 21.1,2Restylane® Refyne was approved for the treatment of moderate to severe facial wrinkles and folds and Restylane® Defyne for the treatment of moderate to severe, deep facial wrinkles and folds.1,2 These scientifically-advanced gels are manufactured with XpresHAn Technology (pronounced ex-spre-shn), creating gels that offer a range of flexibility and support3,4,5 for varied patient needs. Restylane® Refyne and Restylane® Defyne have been shown to maintain effectiveness for the treatment of laugh lines for up to 12 months.1,2

"Even with current approved options, many of my patients are still looking for different solutions to treat their laugh lines," said Boca Raton-based oculoplastic surgeon and Restylane® Refyne clinical investigator Steven Fagien, MD. "These new products are flexible and are designed to meet different patient needs. I am excited to offer these next-generation hyaluronic acid (HA) dermal fillers in my practice."
"Restylane® Refyne and Restylane® Defyne are the latest FDA-approved advancements in HA dermal fillers and align with Galderma's mission to help individuals achieve natural-looking results through treatments with a long-standing history of proven safety and efficacy," said Kelly Huang, PhD - VP & General Manager of the U.S. Aesthetic and Corrective business of Galderma. "We saw an opportunity to address a common concern for patients who have not yet tried a dermal filler by designing gels that provide natural-looking results. With these new brands, the Restylane® family of products now represents the broadest offering of HA dermal fillers in the U.S."
The FDA approval was based on two pivotal, double-blinded, randomized, active-controlled Phase 3 studies investigating Restylane® Refyne and Restylane® Defyne (involving 171 and 162 subjects, respectively) to evaluate their safety and effectiveness. In both studies, Restylane® Refyne and Restylane® Defyne met the studies' endpoints, with both products showing a clinically meaningful improvement in wrinkle severity for up to 12 months in the majority of patients. Study investigators used the Wrinkle Severity Rating Scale (WSRS), a validated 5-point measure of the size and depth of the wrinkles, with grade 1 defined as absence of wrinkles and grade 5 as extremely deep and long wrinkles. Investigators reported that 79% of Restylane® Refyne subjects and 77% of Restylane® Defyne subjects had at least a 1-grade improvement on the WSRS after 6 weeks. Subjects also performed self-assessments (SSA) of wrinkle severity, with most subjects reporting at least a 1-grade improvement in SSA scores with Restylane® Refyne and with Restylane® Defyne after 6 weeks.1,2
"Many of my patients are interested to learn about the latest products that can help them achieve natural-looking results, but are oftentimes unsure about starting dermal fillers," said San Diego-based board-certified dermatologist Mitch Goldman, MD. "The introduction of these next-generation HA dermal fillers with XpresHAn Technology has the potential to change my patients' views on fillers. Restylane® Refyne and Restylane® Defyne provide options for patients who want to make sure they can achieve natural-looking results, which is a key need my patients express every day."
After initial treatment, injection site responses (redness, swelling, bruising, lump/bump formation, pain/tenderness) were predominantly mild or moderate in intensity, temporary (typically with a duration of one to two weeks), and similar for the Restylane® products.
XpresHAn Technology
Restylane® Refyne and Restylane® Defyne were first approved in Europe in 2010 under the brand name Emervel® and have a proven safety profile6 demonstrated by more than one million treatments worldwide.7Restylane® Refyne and Restylane® Defyne are dermal fillers injected under the (facial) skin. Once in place, they help smooth away your "laugh lines" the wrinkles and folds that may form at the sides of your nose and run down toward the corners of your mouth. These next-generation dermal fillers were designed using a unique manufacturing process called XpresHAn Technology, which creates a smooth, injectable gel that can give your skin natural-looking results. XpresHAn Technology customizes the degree of HA crosslinking in each product, resulting in gels with a range of flexibility and support characteristics for different patient needs. Restylane® Refyne is designed to be very flexible and provide subtle support, while Restylane® Defyne is designed to be less flexible and provide additional support.
Phase 4 Studies
Galderma is conducting three ongoing Phase 4 clinical studies to investigate the effects of Restylane® Refyne and Restylane® Defyne as patients conduct everyday expressions and is looking forward to sharing these results as they become available.
About Restylane
With over 30 million treatments worldwide and counting,8 the Restylane® line of hyaluronic acid fillers is used to help smooth away wrinkles (Restylane® & Restylane®Lyft), create fuller and more accentuated lips (Restylane®Silk), and add lift and volume to the cheeks (Restylane®Lyft). All Restylane® products work to enhance facial features and give long-lasting, yet non-permanent results.
To learn more about the Restylane® line of products, visit www.RestylaneUSA.com.
About Galderma
Created in 1981, Galderma is now present in over 100 countries with an extensive product portfolio of medical solutions to treat a range of dermatological conditions. The company partners with health care professionals around the world to meet the skin health needs of people throughout their lifetime. Galderma is a leader in research and development of scientifically-defined and medically-proven solutions for the skin, hair and nails.
Strategic brands in the U.S. include Epiduo® Gel, Epiduo® Forte Gel, Oracea® Capsules, Clobex® Spray, Differin® Gel, Mirvaso® Gel, MetroGel® Gel, Soolantra® Cream, Vectical® Cream, Tri-Luma® Cream, Cetaphil®, Benzac® Acne Solutions, Restylane®, Restylane® Silk, Restylane® Lyft, Dysport® and Sculptra® Aesthetic.
For more information, please visit www.galdermausa.com and www.galderma.com.
All trademarks are the property of their respective owners.
To earn exclusive rewards, bonuses and discounts on Galderma's aesthetic treatments, join the ASPIRE Galderma Rewards program. To learn more about ASPIRE, visit www.aspirerewards.com.
Important Safety Information
The Restylane family of products includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Refyne, and Restylane® Defyne.
APPROVED USES
Restylane®and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane®and Restylane-L®are also indicated for injection into the lips in patients over the age of 21.
Restylane® Lyftwith Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies in patients over the age of 21.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles in patients over the age of 21.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds, in patients over the age of 21.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds, in patients over the age of 21.
Are there any reasons why I should not use products within the Restylane® family? (Contraindications)
To ensure a safe procedure, your doctor will talk to you about your medical history to determine if you are an appropriate candidate for treatment. You should not use products within the Restylane family if:
- You have severe allergies with a history of severe reactions (anaphylaxis)
- You are allergic to lidocaine or to any of the gram-positive bacterial proteins used to make hyaluronic acid
- You are prone to bleeding or have been diagnosed with a bleeding disorder
Are there other precautions that I should discuss with my doctor?
- Tell your doctor if you are breastfeeding, pregnant, or trying to become pregnant. The safety of these products for use during pregnancy, or in women who are breastfeeding, has not been studied
- Restylane, Restylane-L, Restylane® Lyftwith Lidocaine, Restylane Refyne and Restylane Defyne are intended to treat facial wrinkles and folds, such as nasolabial folds. Restylane and Restylane-L are also intended for lip enhancement. Treatments in other areas of the face have not been evaluated in clinical studies.
- The safety and effectiveness of Restylane® Silk for areas other than the lips and perioral area have not been evaluated in clinical studies
- Tell your doctor if you have any history of scarring, particularly thick and stiff scars, or any pigmentation (skin color) disorders. These side effects can occur with hyaluronic acid fillers in general.
- Tell your doctor if you are planning other laser treatments or a chemical peel, as there is a possible risk of inflammation at the treatment site if these procedures are performed after treatment
- Patients who experience skin injury near the site of injection with these products may be at a higher risk for side effects
- Tell your doctor if you are on any medications to decrease your body's immune response (immunosuppressive therapy). Using these medications may increase your risk of bruising or bleeding at the gel injection site.
- Tell your doctor if you are using any "blood thinners" such as aspirin, warfarin, or any other medications that affect bleeding. Using these medications may increase your risk of bruising or bleeding at the gel injection site.
- The use of these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete. Use of product in these areas could delay healing or make your skin problems worse.
What are the possible side effects?
The most commonly observed side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, and itching at the injection site. These are typically mild in severity and typically resolve in less than 7 days in nasolabial folds and less than 14 days in lips. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site.
One of the risks with using this product is unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious, and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin.
As with all skin injection procedures, there is a risk of infection.
To report a side effect with any of the Restylane products, please call Galderma Laboratories, L.P at 1-855-425-8722.
The Restylane family of products is available only through a licensed practitioner. Complete Instructions for Use are available at www.RestylaneUSA.com.
1 Galderma. Restylane® Refyne Instructions for Use. 2016.
2 Galderma. Restylane® Defyne Instructions for Use. 2016.
3 A pivotal USA randomized evaluator-blinded, active-controlled, multi-center, split-face comparison study of Emervel® Deep Lidocaine versus Juvederm® Ultra Plus in the treatment of moderate to severe facial wrinkles and folds [Phase 3 Support Study: RD.06.CIR.18159 CSR Emervel Deep, phase 3 vs Juvederm Ultra Plus, 79w]
4 A pivotal USA randomized evaluator-blinded, active-controlled, multi-center, split-face comparison study of Emervel® Classic [Phase 3 Support Study: RD.06.CIR.18156 CSR Emervel Classic, phase 3 vs Juvederm, 76w]
Lidocaine versus Juvederm® Ultra in the treatment of moderate to severe facial wrinkles and folds
5 Evaluation of amplitude sweep cross-over point as an index of flexibility [MA-32418 Final Flexibility Report], October 4, 2016.
6David E. Bank, MD, Derek H. Jones, MD, Cindy Wong, MD. A Comprehensive Analysis of the Safety of a New Range of Injectable Hyaluronic Acid Products for Aesthetic Indications. For publication in ASDS program. November 2016.
7 DOF. Galderma Laboratories, L.P. [Global Launch Learnings]
8 DOF. Galderma Laboratories, L.P. [Claim Substantiation - Broadest Range of Fillers]. September 2016.





To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/galderma-announces-fda-approval-of-restylane-refyne-and-restylane-defyne-dermal-fillers-for-treatment-of-laugh-lines-300376359.html
SOURCE Galderma





