Frontier Biotechnologies, announced positive results from the Phase 1 clinical trial of its drug candidate, FB2001 – a small molecule inhibitor of coronavirus main protease (Mpro) – in healthy adult volunteers.
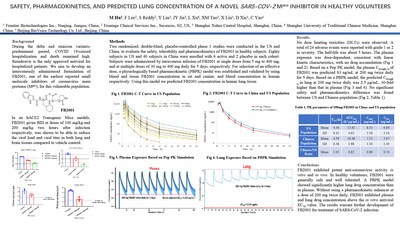
ATLANTA, Aug. 8, 2022 /PRNewswire/ -- Frontier Biotechnologies, a biopharmaceutical company focused on the discovery, development and dissemination of innovative medicines that improve patient health, announced positive results from the Phase 1 clinical trial of its drug candidate, FB2001 – a small molecule inhibitor of coronavirus main protease (Mpro) – in healthy adult volunteers. The data, presented today at the poster session of the 11th International Conference on Emerging Infectious Diseases (ICEID), showed FB2001 to be safe and well tolerated among trial participants. Adverse events reported during the trial were mostly mild-to-moderate in severity, with no significant differences observed between participants in the Chinese and American study centers. "We are pleased by the positive results from FB2001's phase 1 trial. It is a significant milestone for us and the healthcare community", said Dr CJ Wang, Chief Executive Officer of Frontier Biotechnologies. "This promising result will spur us on to strive for success in later-stage trials. We believe that the work done at Frontier Biotech can elevate our efforts in the fight against COVID-19 in China and abroad." A total of 120 participants (80 Whites in the US and 40 Chinese in China) received intravenous infusions of FB2001 at either single doses from 5 mg to 400 mg, or multiple doses of 30 mg to 400 mg daily for 5 days. The key findings from the study are as follows:
The results from the Phase 1 trial build on preclinical in vivo data of FB2001, where the drug was observed to reduce viral loads in both the lung and brain tissues of mice. Pharmacokinetic data obtained from preclinical studies showed significantly higher concentration of FB2001 in the lung compared to plasma. "FB2001 has demonstrated in vivo antiviral activity in the lung and brain tissue of SARS-CoV-2 mouse model without the need for pharmacokinetic boosting. Therefore, it holds great promise as a treatment for acute COVID-19 as well as long-COVID, both of which will be evaluated in further follow-up studies", said Dr Jay Lalezari, MD, Medical Director of Quest Clinical Research in San Francisco. The current intravenous formulation of FB2001 is ideal for hospitalized patients with its rapid onset of action and is suited for patients with dysphagia or other problems with swallowing. Working with clinical research organizations, regional regulatory agencies, and local clinical centers, Frontier Biotechnologies has started a pivotal Phase 2/3 study (BRIGHT trial) to enroll about 1,200 hospitalized patients in hundreds of clinical centers worldwide. "The phase 1 data were really promising", said Dr Michael Hu, Chief Medical Officer of Frontier Biotechnologies, "and we are confident to carry out the pivotal trial to explore the utility of the drug in reducing the time to recovery in hospitalized patients due to COVID-19." Frontier Biotechnologies is also developing a pulmonary formulation of FB2001 that could be used in out-patient setting for the treatment of mild Covid-19, as well as for post exposure prophylaxis. When inhaled directly into the respiratory tract and lungs, the tissue concentration of FB2001 is much higher than that in plasma; hence, the onset of action and viral clearance could potentially be faster than that of oral therapy. About Frontier Biotechnologies Founded in 2013, Frontier Biotechnologies Inc. ("Frontier Biotech") is a commercial-stage biopharmaceutical company headquartered in China with global vision and world-class competitiveness. Frontier Biotech is committed to discovering, development, manufacturing, and commercialization of innovative medicines that improve patient health.
SOURCE Frontier Biotechnologies |