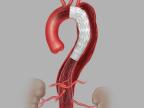
Following the recent FDA approval of Cook Medical’s Zenith Dissection Endovascular System, Cooper University Health Care in Camden, New Jersey, has treated the first patient in the US with the device as part of Cook’s US commercial launch.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190328005864/en/
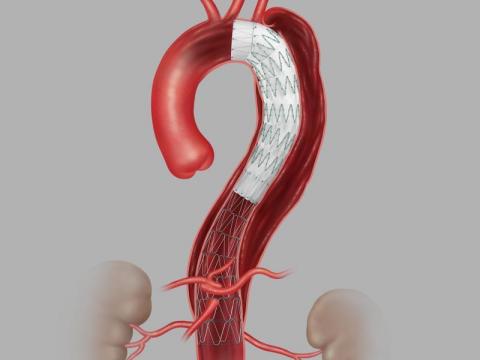
Zenith® Dissection Endovascular System (Graphic: Business Wire)
“We are committed to helping patients by developing a variety of treatment options for aortic disease,” said Mark Breedlove, vice president of Cook Medical’s Vascular division. “We’re pleased to provide US clinicians and patients another minimally invasive option for aortic repair.”
The system provides physicians a less invasive alternative to open surgery for repair of Type B dissections of the descending thoracic aorta. It consists of a proximal stent-graft component and a distal bare stent component.
“The value in this dissection stent is that it’s pathology-specific, designed just for this disease," said Joseph Lombardi, M.D., director of the Cooper Aortic Center. “As the principal investigator, receiving FDA approval is something that I had really looked forward to. It’s really exciting and I think it’s going to make a big impact on how dissection is managed.”
Aortic dissection is a tear that occurs between the innermost and middle layers of the aorta. When the inner layer of the aorta tears, blood flows through the tear, which causes the inner and middle layers of the aorta to separate (dissect). Type B dissection involves a tear in the descending aorta.
Globally, thoracic endovascular aortic repair (TEVAR) is acknowledged as the treatment of choice for complicated Type B aortic dissection. These procedures are meant to prevent malperfusion of aortic branches and aortic rupture.
To learn more about Cook’s disease-specific treatment options for endovascular repair, visit cookmedical.com.
Dr. Lombardi is the Global Principal Investigator of the Stable I and Stable II Clinical Trials and is a paid consultant of Cook Medical.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20190328005864/en/
Contacts
Moriah Sowders
External Corporate Communications Generalist, Cook Medical
812.340.4347 (mobile)
812.339.2235, ext. 10-4357 (office)
Moriah.Sowders@CookMedical.com
Source: Cook Medical