Fidmi Medical Ltd. a portfolio company of The Trendlines Group Ltd. (“Trendlines”) (SGX: 42T) (OTCQX: TRNLY), announced today that it received 510K regulatory clearance from the United States Food and Drug Administration (FDA) for its low-profile enteral feeding device.
|
CAESAREA, Israel, Oct. 16, 2019 /PRNewswire/ -- Fidmi Medical Ltd. a portfolio company of The Trendlines Group Ltd. ("Trendlines") (SGX: 42T) (OTCQX: TRNLY), announced today that it received 510K regulatory clearance from the United States Food and Drug Administration (FDA) for its low-profile enteral feeding device.
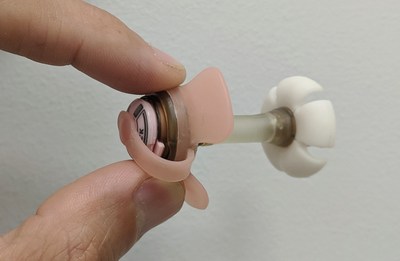
Gastrostomy tubes, which help patients receive long-term nutritional support, are typically placed endoscopically and need to be replaced every 3-6 months. Complications such as clogging and dislodgement are common, resulting in inadequate nutrition and medication delivery, numerous tube replacements, hospitalizations, and a substantial financial drain on patients and the health care system. Fidmi Medical's innovative low-profile gastrostomy system is unique in that it can be utilized for both initial placement and replacement and has several features which make it more durable and comfortable for patients. Gastrostomy tubes very often get dislodged or clogged, promoting infection, and need to be replaced frequently. Fidmi's improved low-profile gastrostomy tube is placed just like any standard Percutaneous Endoscopic Gastrostomy (PEG) tube but has an easily replaceable inner tube which can be changed by patients without the need to re-enter the healthcare system for replacement procedures. This will result in fewer complications with patients' gastric tubes, therefore potentially reducing healthcare costs for payers and healthcare systems; as well as providing a substantial improvement in quality of life for patients and their caregivers. Fidmi Medical CEO Shahar Millis remarked, "Receiving FDA clearance is a remarkable milestone for us. FDA clearance allows us to enter the US market; to provide improved patient quality of life and impact the ease, efficiency and confidence of clinicians' work." This is the second important milestone in two months, in September Fidmi announced their collaboration with CoapTech LLC from Baltimore, MD to allow a broader solution to tube-supported patients. About Fidmi Medical Fidmi Medical is an Israeli company founded in 2014, dedicated to developing enhanced feeding devices that offer easy insertion, replacement, and removal. The Company was founded with investment and support of The Trendlines Group’s medtech incubator, and support from the Israel Innovation Authority. Fidmi Medical is currently raising a new investment round to bring the company to commercialization. Contact: Photo - https://mma.prnewswire.com/media/1011855/Fidmi_Medical_PEG_Tube.jpg
SOURCE Fidmi Medical Ltd. |
||
Company Codes: OTC-PINK:TRNGF, OTC-PINK:TRNLY, Singapore:42T, OTC-QX:TRNLY, OtherOTC:TRNLY |