Tidmarsh, an adjunct professor at Stanford’s medical school, brings decades of industry experience to the table. Serving as director of the Center for Drug Evaluation and Research will be his first government position.
George Tidmarsh, an adjunct professor of pediatrics and neonatology at Stanford University’s School of Medicine with a long history in the biopharma and biotechnology industries, will be the FDA’s next head of the Center for Drug Evaluation and Research.
According to the FDA’s announcement of his appointment Monday, Tidmarsh has been involved in the “successful clinical development of seven FDA-approved drugs.”
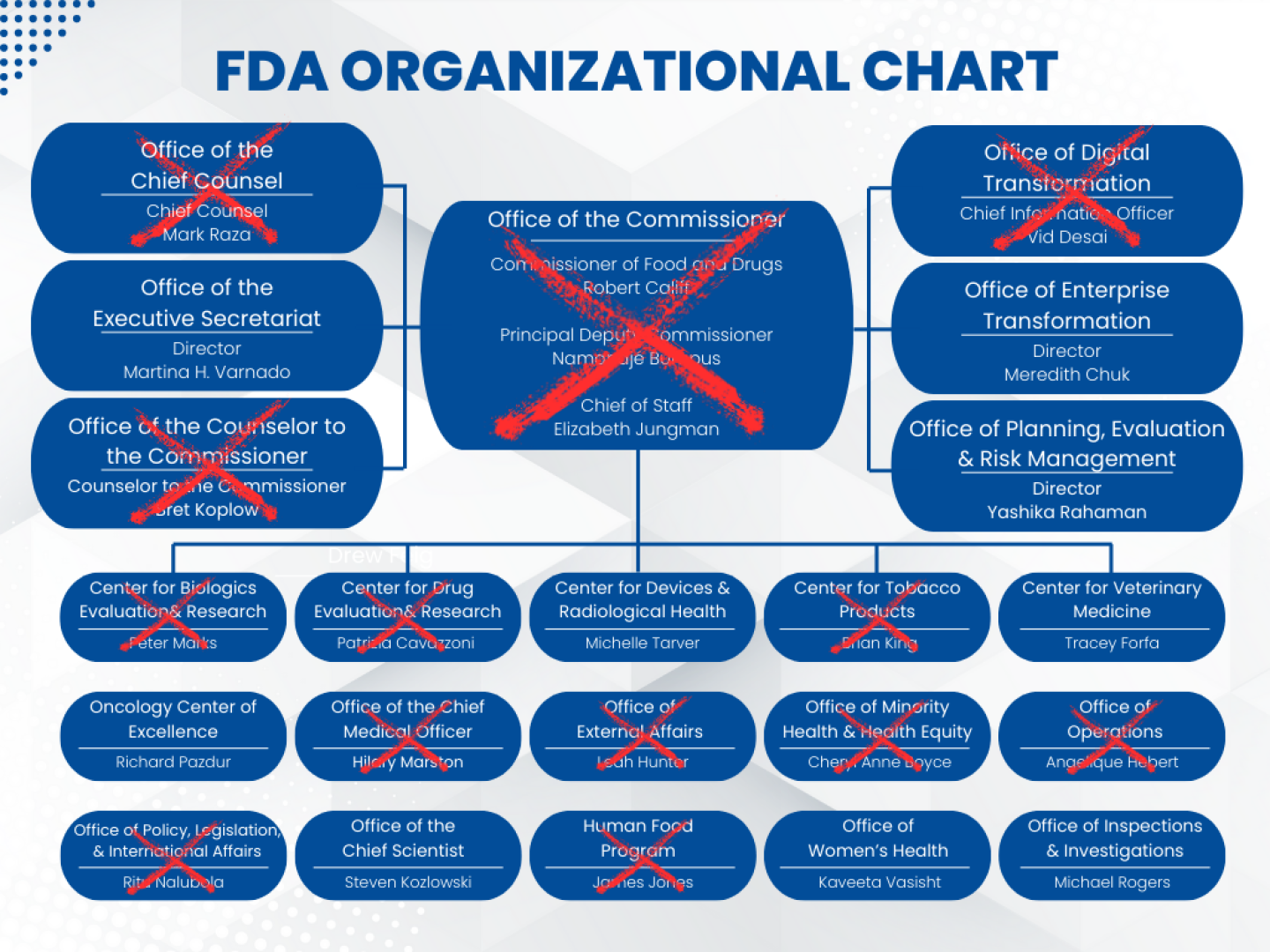
Tidmarsh is replacing Jacqueline Corrigan-Curay, the current acting head of CDER. Corrigan-Curay announced in June that she would be departing in July. She herself had only been in the job since January when Patricia Cavazzoni left the position two days before President Donald Trump began his second term.
Tidmarsh’s hire highlights a much-debated issue of the FDA’s so-called revolving door with industry. Until May, Tidmarsh was the chairman of Revelation Biosciences, a Southern California–based startup he cofounded focused on kidney conditions and treatments for surgical infections. Before that Tidmarsh had been an executive at a suite of other companies, including stints as the CEO of Solano Therapeutics, La Jolla Pharmaceutical Company and Horizon Pharma, another company he founded. Horizon was purchased by Amgen in 2023 for nearly $28 billion, giving the larger company control of the seven FDA-approved drugs that Tidmarsh had a hand in. Those include rare disease drugs like Uplizna, Krystexxa and Tepezza. Sales of those drugs have been coming up short of expectations for Amgen since the buy.
“I look forward to working with [Tidmarsh] to strengthen our drug review programs, foster innovation, and advance cross-agency initiatives that improve health outcomes for the American public,” FDA commissioner Marty Makary said in the agency’s statement.
Tidmarsh has no previous experience working in government or regulation. His predecessors, Corrigan-Curay and Cavazzoni, both started at lower level appointments at the FDA. Corrigan-Curay had nine years of experience before taking the top CDER job, while Cavazzoni had about three and a half years.
Though he has no previous government experience, Tidmarsh has appeared as an expert scientist before FDA panels convened to discuss the food additive talc. That could potentially overlap with overview of drugmaker Johnson & Johnson, whose talc-based powder products have been at the center of a 16-year legal battle.
Tidmarsh’s appointment is the latest in a rush of new names in at the FDA as more and more leaders exit the agency. At CDER’s sister regulatory the Center for Biologics Evaluation and Research, longtime head Peter Marks was pushed out earlier this year and University of California, San Francisco professor and YouTube commentator Vinay Prasad brought on in his stead. Once in office, Prasad pushed out the agency’s top cell and gene therapy regulator Nicole Verdun, as well as her deputy Rachael Anatol.
All told, fewer than half of senior FDA leaders from six months ago remain at the agency today.