Drug Development
Infigratinib topped “even the most optimistic expectations” for efficacy and safety in the late-stage PROPEL 3 study in achondroplasia, Truist Securities analysts said Thursday.
FEATURED STORIES
Analysts, investors and scientists are eager for Biogen’s 2026 BIIB080 readout. Even if successful, executives warn that there are many more steps before the Alzheimer’s therapy could reach the market.
With a clutch of key data and planned regulatory applications this year from Avidity Biosciences, REGENXBIO and Capricor Therapeutics, CureDuchenne CSO Michael Kelly sees “momentum” in the Duchenne muscular dystrophy pipeline, as Sarepta’s Elevidys leaves the door open.
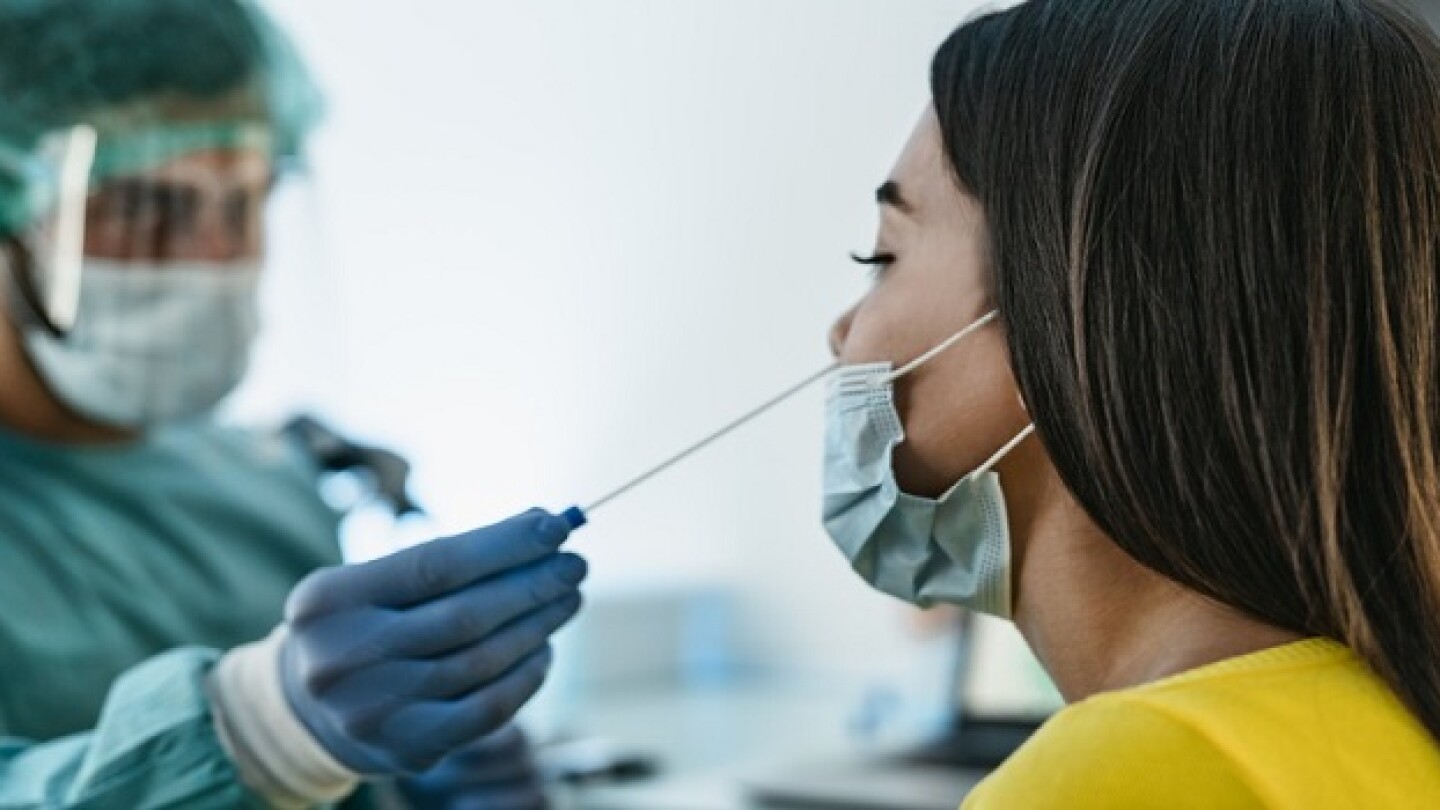
After advancing in lockstep through the pandemic, the fortunes of the biotechs have diverged as their use of COVID-19 windfalls has taken shape.
Subscribe to ClinicaSpace
Clinical trial results, research news, the latest in cancer and cell and gene therapy, in your inbox every Monday
THE LATEST
Avadel and Jazz Pharma, a leader in treating sleep disorders, are locked in a patent dispute. If it receives final FDA approval, Avadel’s Lumryz could challenge Jazz’s market dominance.
Sanofi has terminated a development agreement with Regulus in Alport syndrome, while Merck reaches the point of futility in a Phase III colorectal cancer trial.
As the BA.4/BA.5 Omicron subvariants spread, researchers continue to develop COVID-19 therapies and approaches for preventing or treating the disease. For that and more, continue reading.
Innovent Biologics’ Phase II trial of mazdutide, a potential treatment for type 2 diabetes, showed the drug met its primary endpoint by successfully reducing HbA1c levels.
The FDA has approved Incyte’s Opzelura as the first and only at-home therapy for repigmentation in nonsegmental vitiligo.
The buyout comes on the heels of promising Phase I/II results from GTX-102, an antisense oligonucleotide candidate being developed to treat Angelman syndrome.
In what it is calling a strategic decision, Sesen Bio announced Monday that it has paused development activities of Vicineum, its lead asset, in the United States.
In the latest in Nestlé’s efforts to establish a presence in the gastroenterology space, the company will pay over $40 million upfront to Enterome as part of a new partnership.
MacroGenics’ Phase II trial shuts down after a patient death believed to be related to enoblituzumab combo, while Exelixis’ Cabometyx combo improves PFS in renal cell carcinoma.
Researchers point out that antibodies against amyloid are still considered an essential approach to treating Alzheimer’s, but the leading risk factor for sporadic AD is aging.