Drug Development
Infigratinib topped “even the most optimistic expectations” for efficacy and safety in the late-stage PROPEL 3 study in achondroplasia, Truist Securities analysts said Thursday.
FEATURED STORIES
Analysts, investors and scientists are eager for Biogen’s 2026 BIIB080 readout. Even if successful, executives warn that there are many more steps before the Alzheimer’s therapy could reach the market.
With a clutch of key data and planned regulatory applications this year from Avidity Biosciences, REGENXBIO and Capricor Therapeutics, CureDuchenne CSO Michael Kelly sees “momentum” in the Duchenne muscular dystrophy pipeline, as Sarepta’s Elevidys leaves the door open.
After advancing in lockstep through the pandemic, the fortunes of the biotechs have diverged as their use of COVID-19 windfalls has taken shape.
Subscribe to ClinicaSpace
Clinical trial results, research news, the latest in cancer and cell and gene therapy, in your inbox every Monday
THE LATEST
BrainStorm Cell Therapeutics said it will submit a Biologics License Application to the FDA for ALS hopeful NurOwn. Additionally, a correction has been made to analyses of the Phase III clinical trial.
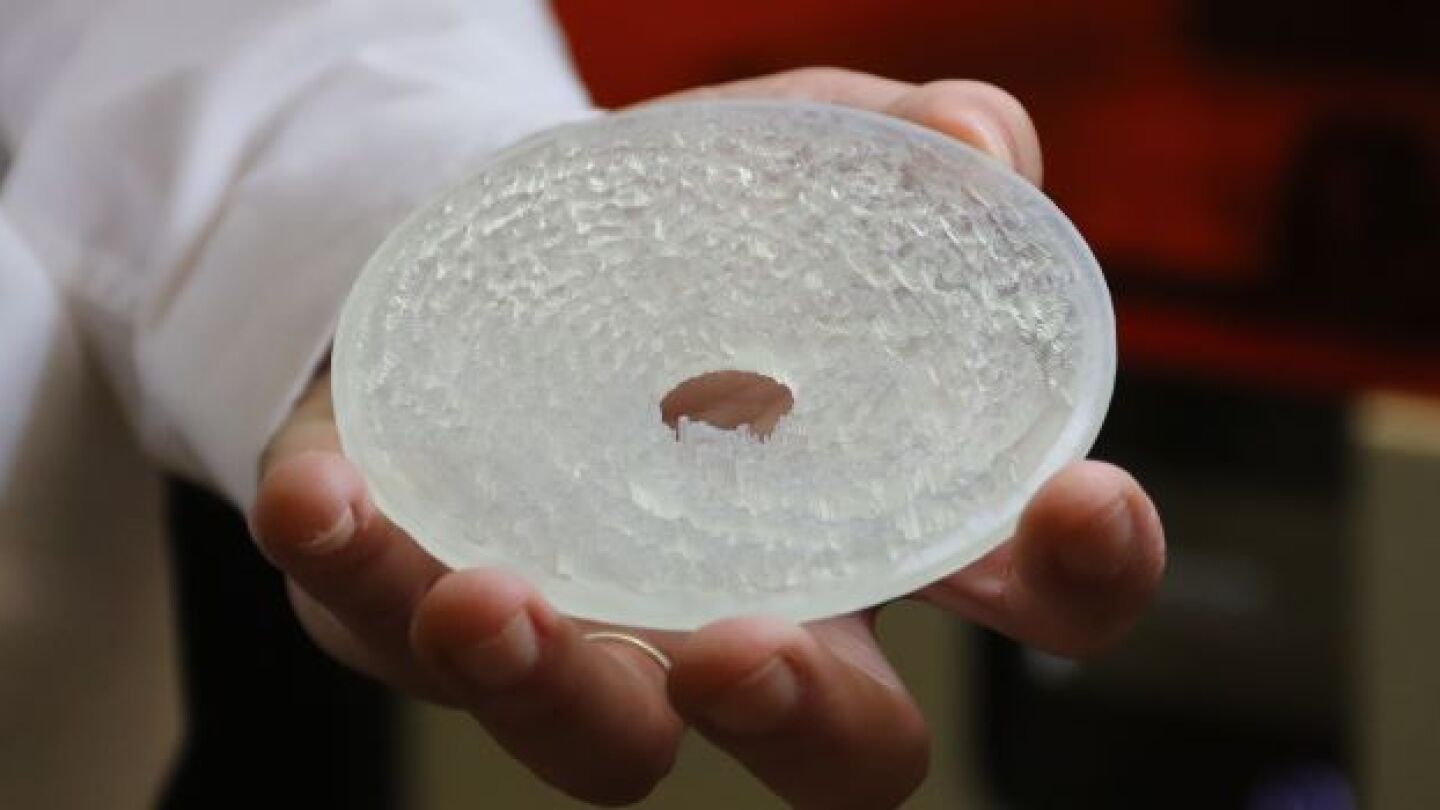
The 3D-printed acoustic hologram could be very important to the delivery of certain therapeutics for Alzheimer’s Disease (AD) and other neurological disorders.
This week holds moments of truth for Provention’s type 1 diabetes drug and bluebird bio’s gene therapy for beta-thalassemia. Amicus and Incyte are coming up at the end of the month.
Roche Wins Approval for Flu Drug in Young Children, Pfizer Touts Positive Results for Infant Vaccine
Roche and Pfizer shared positive news from their respective forays into a new pneumococcal vaccine for infants and a treatment for influenza in small children.
AstraZeneca and Daiichi Sankyo’s Enhertu has been approved by the FDA as the first HER2-directed medicine for the treatment of patients with HER2-mutant metastatic NSCLC.
Amgen announced two lung cancer studies with mixed results, Innovent dosed the first patient in a Phase I diabetic macular edema study and HUTCHMED hit the primary endpoint in colorectal cancer.
Centessa halted ZF874 following the report of an adverse event that involved elevated liver enzymes in a Phase I study. This is the second development program the company has stopped in as many months.
A new study on Alzheimer’s disease ties the brain’s immune response to the condition, while scientists have identified a better antibody treatment for cancer and more research news.
Panacell Biotech plans to initiate a program to investigate the use of natural killer (NK) immune cells, exosomes and brown adipose-derived stem cells to treat Long COVID.
Vyne Therapeutics’ combination therapy for atopic dermatitis failed to meet the primary endpoint in a Phase II trial, leaving the company evaluating its priorities and pipeline.