As AAV9 and CRISPR programs navigate safety, delivery and scalability hurdles, small molecules offer a deployable, scalable bridge, complementing genetic approaches and accelerating meaningful impact for patients with Duchenne muscular dystrophy.
The pursuit of a cure for Duchenne muscular dystrophy has long inspired some of the boldest scientific efforts in rare disease medicine. Fueled by urgency, innovation and hope, the field has made remarkable strides—from gene therapy to gene editing and beyond. Yet despite this promise, recent clinical setbacks remind us that no single modality will solve DMD. To truly deliver for patients, we must broaden our therapeutic toolkit, bringing forward small molecules, cell therapies and advanced biologics to complement, not replace, genetic approaches.
As CEO of Sarcomatrix Therapeutics, I lead a team developing small molecule therapies for muscle-wasting diseases, including DMD. Based on my decades of experience in biotech, it is clear to me that small molecules deserve a seat alongside genetic and cell-based approaches in the battle against this formidable foe.
Diversifying the Vector Toolbox
First, let’s address gene therapy. Over the last several years, much of the field has rallied around AAV9-based gene therapy as a potential breakthrough for DMD. AAV9 vectors have clear strengths: they effectively target muscle tissue, cross the blood-brain barrier and enable systemic delivery of therapeutic genes such as microdystrophin. Companies like Pfizer, Sarepta and Solid Biosciences have led ambitious efforts in this space. But tragic clinical events—including multiple patient deaths across trials and with approved drugs—have revealed the limitations and risks of high-dose systemic AAV delivery in this population.
While AAV9 has dominated the DMD gene therapy landscape, it is not the only option. Other adeno-associated virus serotypes, such as AAV8 and AAVrh74, offer different tissue tropism profiles and may improve transduction efficiency or safety in certain contexts. AAVrh74, for example, has shown promising muscle-targeting characteristics with potentially lower immunogenicity and is being evaluated in experimental gene therapies for multiple neuromuscular diseases. Sarepta’s other AAVrh74-based programs—including SRP-9003 for LGMD2E and SRP-9004 for LGMD2D—remain in clinical trials.
Real progress in DMD will likely require synergy, not singularity.
Beyond AAV vectors, lentiviral vectors—though more commonly used ex vivo—also represent another avenue for therapeutic delivery in select applications. And beyond viral systems altogether, several groups are advancing non-viral delivery platforms that may eventually bypass some of the safety and dose limitations of AAV.
Lipid nanoparticles (LNPs), already clinically validated in mRNA vaccines, are being engineered for nucleic acid delivery to muscle. Polymeric nanoparticles, peptide-based delivery vehicles and exosome-like vesicles are also under active investigation. These technologies could enable redosing, reduce manufacturing bottlenecks and allow for modular payloads—including CRISPR components—without the long-term persistence associated with viral genomes.
None of these alternatives are without their own challenges—whether it’s tissue targeting, endosomal escape or large-scale manufacturing—but diversifying the vector toolbox will be critical to building a sustainable gene delivery ecosystem for DMD.
The ‘Trough of Disillusionment’
AAV9 isn’t inherently flawed—but it may not be sufficient on its own. Its use in DMD has shown the strain of trying to stretch a platform beyond its intended scope. We are asking it to transduce nearly every muscle in the body, across variable disease stages, in patients who often have pre-existing cardiac involvement and immune priming. This is a tall order for any delivery system.
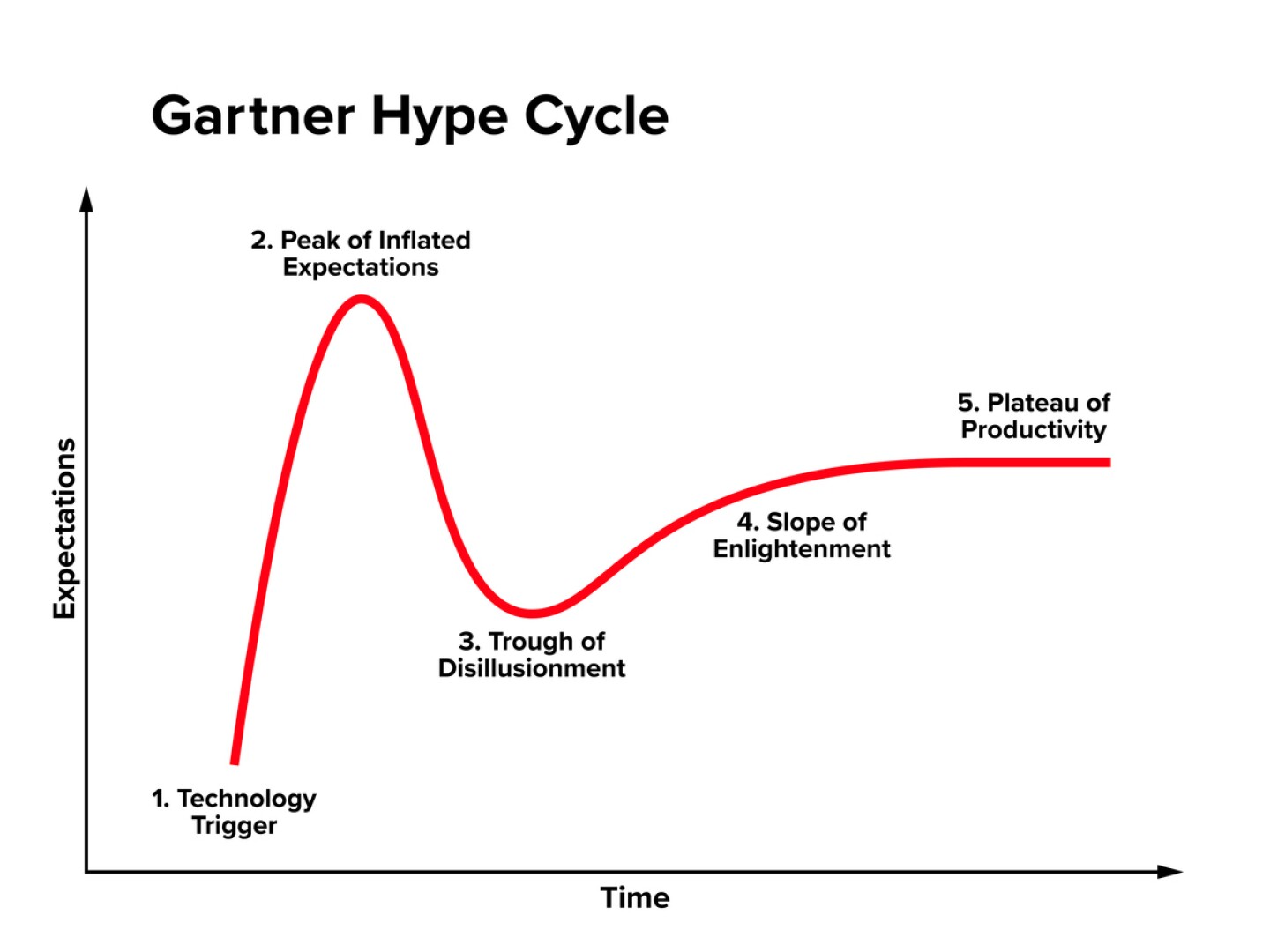
This moment represents a familiar stage in the Gartner Hype Cycle, a model used to describe the trajectory of emerging technologies. Gene therapy for DMD has crested the “Peak of Inflated Expectations” and now enters the “Trough of Disillusionment,” a phase where the technology’s promise is tested by real-world complexity, safety concerns and scalability challenges.
This is not failure—it’s evolution. It’s an opportunity to step back, reassess and expand our approach.
A similar trajectory played out with CAR T cell therapies in oncology. When early trials in refractory B cell leukemias and lymphomas reported unprecedented remission rates, CAR T was vaulted to the “Peak of Inflated Expectations.” Investor capital poured in, pipelines swelled and the technology was hailed as a paradigm-shifting cure. But real-world complexity quickly tempered the hype: severe toxicities like cytokine release syndrome and neurotoxicity emerged, efficacy in solid tumors proved elusive, and the logistical and manufacturing challenges of autologous cell therapy became clear. The field entered its “Trough of Disillusionment,” marked by program pauses, trial redesigns and more selective patient targeting.
Through iterative advances in safety management, antigen targeting and manufacturing, CAR T has now moved into the “Slope of Enlightenment” with multiple approvals, broader indications and a more sustainable integration into cancer care.
iStock, PeterHermesFurian
Small Molecules—A Bridge to a Cure
For patients with DMD, small molecules offer a critical, proven near-term bridge to the future. While gene editing may one day deliver a lasting solution, it’s still in its formative stages, and systemic delivery remains a limiting factor. In contrast, small molecule therapeutics are already deployable, scalable and well understood from both regulatory and clinical perspectives. These compounds can target pathways critical to muscle health—such as inflammation, regeneration, fibrosis and integrin signaling—while avoiding the risks associated with irreversible genome editing or immune activation.
Oral small molecules can be titrated, paused and combined with other therapies. They are especially promising as a baseline treatment for all patients, including those not eligible for gene therapy or CRISPR trials. In this way, small molecules serve not as a competitor to gene editing but as a complement and a bridge, offering meaningful clinical impact while the field continues to mature more permanent and personalized therapies.
Cell therapies also hold enormous potential. Satellite cell transplantation, stem cell–derived myoblasts and engineered muscle progenitors are making early clinical progress. These approaches may help rebuild damaged tissue or deliver supportive trophic factors—especially when used in tandem with pharmacological or genetic tools.
And of course, gene editing platforms like CRISPR remain powerful. But they are also early. Delivery challenges persist, and systemic editing of muscle cells across the body is still years from being fully optimized. Most CRISPR-based programs still rely on AAV vectors for in vivo delivery—bringing us back to the same issues of dose, immunogenicity and off-target exposure.
The future of DMD treatment isn’t about choosing between gene therapy or small molecules, editing or exon-skipping. It’s about combining these tools in thoughtful, patient-centered ways. Some patients may benefit from gene transfer early in life. Others may rely on a foundation of small molecule therapies to delay progression or complement other interventions. Still others, especially those with cardiac involvement, may need targeted, organ-specific solutions.
Regulators, researchers and investors must embrace this complexity. A one-size-fits-all approach is no longer viable. We must fund and advance multiple modalities in parallel, recognizing that each brings unique advantages—and that real progress in DMD will likely require synergy, not singularity.
DMD patients and their families have waited long enough. It’s time we stop searching for the one solution and instead commit to building a comprehensive arsenal of tools to treat—and ultimately overcome—this devastating disease.