CORD, LLC, a privately held ophthalmic medical device company, announced today that it has submitted a Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA) for the Model SC9 Intraocular Lens (IOL) for the treatment of cataracts.
|
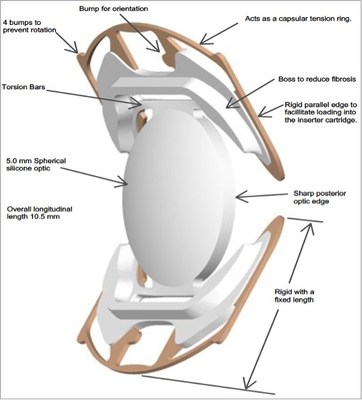
SC9 Incorporates 21 Patented Features Designed to Position the Lens for Superior Intermediate Vision ALISO VIEJO, Calif., Dec. 8, 2022 /PRNewswire/ -- CORD, LLC, a privately held ophthalmic medical device company, announced today that it has submitted a Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA) for the Model SC9 Intraocular Lens (IOL) for the treatment of cataracts. The SC9 is the brainchild of Stuart Cumming, MD, a pioneer in IOL research and development.
The SC9 is the brainchild of Stuart Cumming, MD, FACS, FRCOphth, who has been at the forefront of technology to improve and enhance vision for more than four decades. He is a pioneer in IOL research and development, with over one hundred patents related to intraocular lenses. Dr. Cumming designed the Crystalens®, approved by the FDA in 2003. The SC9 lens was designed to treat cataract patients with a single focus spherical optic and a rigid structure to consistently locate the optic in a position intended to provide intermediate vision that is superior to that of a standard monofocal IOL. CORD is seeking market approval of the SC9 intraocular lens based on the results of more than 300 patients who were followed for a minimum of one year under an approved Investigational Device Exemption (IDE). ABOUT CORD, LLC AND INVENTOR STUART CUMMING, MD
Cumming Ophthalmic Research and Development (CORD, LLC) is a privately held ophthalmic medical device company founded by Dr. Stuart Cumming, MD. The Model SC9 represents Dr. Cumming's latest breakthrough in vision technology. After patenting and sequentially developing the six iterations of the Crystalens design and conducting clinical trials in Germany, Dr. Cumming co-founded Eyeonics in 1998. The Crystalens was approved by the FDA in November 2003 as the first and only accommodating IOL. Eyeonics was purchased by Bausch + Lomb in 2008. The Model SC9 lens is the result of 28 years of research, coupled with Dr. Cumming's expertise and continued devotion to IOL research and development. Media Contact
SOURCE CORD, LLC |