COSTA MESA, CA--(Marketwire - July 27, 2009) - CNS Response, Inc. (OTCBB: CNSO) provides a Letter to Shareholders from Chief Executive Officer George Carpenter.
"Dear Shareholder,
"If you've been keeping up on CNS Response news lately, you probably have questions. As I've spoken to shareholders over the last couple of months, I've been impressed by the commitment and domain expertise of our shareholder base: many have owned this company for a long time, know its opportunities and pitfalls, and want it to succeed. So it made sense to me to summarize these discussions and outline the challenges we see ahead.
"Three months ago, our position as a company was pretty dire, with obligations greatly exceeding our assets. Some examples:
-- The Company's major multi-site clinical trial (the "Depression
Efficacy trial") hadn't completed recruiting and the Company didn't have
the money to complete it.
-- Vendor payables had been stretched, some for years, and many vendors
looked like they could be moving to termination or litigation.
-- Paid test volume was static, as was the database.
-- The Company had never scheduled an Annual Meeting under Len Brandt.
-- CNS Response was no longer a recognized corporate entity in Delaware,
its state of incorporation, due to failure to pay its corporate franchise
tax.
"After 12 months of unsuccessful financing efforts, Len Brandt presented the Board with his plan: discontinue payroll, notify clinical trial sites that further payments would be delayed or suspended, and use bridge loans to 'drip feed' the company on a month-to-month basis.
"On behalf of shareholders, the Board refused to accept that path. They reasoned that the company could be significantly more valuable with a successfully completed study, a network of trained physicians, an organization to service them and scale with their growth, and adequate capital to avoid running out of cash every two months. The Board made the decision to change leadership and seek additional financing, as opposed to risking bankruptcy and 100% shareholder dilution.
"Accordingly, I was asked on April 10th to pull the team together, complete the clinical trial, and raise enough money to ensure that the trial reached the market with sufficient momentum to generate national attention and commercial reimbursement. We set out three goals for the first 90 days:
1. Complete the Depression Efficacy Trial
-- Situation in April: The biggest single investment the Company has made to date is its current multi-site Depression Efficacy Trial. At the beginning of April, the Company began notifying sites that it wouldn't make payments on past invoices, due to a lack of capital.
-- There was deep concern within the team that sites would terminate -- or slow -- their work with us, jeopardizing completion of the trial.
-- 90-day objective: We resolved to complete recruiting in all sites by June, perform an interim validation analysis to confirm that the study is sufficiently powered, and endeavor to announce study results by year-end.
-- Actions & Results: Full effort has been maintained at all active trial sites. We negotiated extended payment agreements with most sites, and have honored those agreements. Overall, we've reduced payables outstanding to trial sites by 25% and have increased our speed of payment considerably.
- The impact of continuing our study has been good: as all sites have
continued to participate and follow the study protocol, we are
currently on track for a November release of top-line data at the
U.S. Psychiatric Congress, and a solid publication strategy
thereafter.
- Validation study by biostatistician AMAREX was encouraging: our
trial was "powered" at fewer subjects than originally required,
allowing us to end recruiting for the study at 114 subjects on
June 12, 2009.
2. "Monetize the test"
-- Situation in April: During this period, physicians using Referenced-EEG® in their practices continued to report exceptional results for their patients, and many new physicians began training in the use of rEEG®. So the question is, can we grow rEEG testing profitably? The clinical trial is likely to attract significant attention to our technology, but the real proof for investors will be in our ability to monetize the test. Fortunately, there are some good companies who have successfully commercialized similar biomarkers (e.g. Genomic Health), and we're following their well-worn path.
Our estimate is that breakeven cash flow at current expense levels would require over 500 paid tests per month; recent months have been in the 20's and 30's. Our goal is to demonstrate meaningful progress toward closing that gap by the end of 2009.
-- 90-day objective: Get the service team and physician network focused on an achievable goal -- 100 tests per month by 12/09 -- with a limited commercialization budget.
-- Actions & Results
- Listening to our physician partners has produced immediate results.
There's always the question with new medical technologies as to how
widely they will be accepted and adopted by physicians. rEEG is no
different. However, there are a dozen "early adopters" of rEEG who
overcame those obstacles, and we can learn much from them. For the
first time, these users now collaborate on a regular basis with the
company and each other, sharing best practices and product
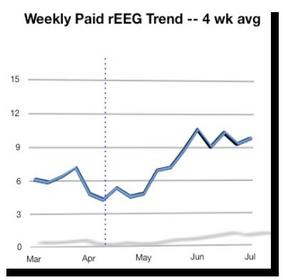
recommendations. (See Graph: 'Weekly Paid rEEG Trend – 4 wk avg.')
We've invested ourselves not only in improving the rEEG test, but in
improving the marketing and logistics of that test inside each
physician practice. And we're finally moving forward with critical
product development projects to enhance our report and database.
- Test volume is up sharply, mostly as a result of focus and attention
on the basics of testing operations with these top physicians.
Nevertheless, the numbers are still small and will require a
sustained effort to achieve our December goal.
- Applying the marketing lessons from our clinical trial: long after
the study was "powered" and recruiting goals were achieved, a
relatively modest investment in web and other media was still
generating referrals. We are beginning to apply many of the same
web-based tools to generate testing volume within our physician
network.
- Management review: we have organized our team to maximize focus
and accomplishment of short-term goals. As part of this process, an
independent management consultant interviewed each member of the
team shortly after the transition. Their report identified
significant improvement in productivity and morale since April.
Shareholders are welcome to contact any of our employees (or
ex-employees) to assess this improvement in management effectiveness
for themselves.
3. Raise $4-5 million in capital
-- Situation: Under Len Brandt's leadership, the Company spent considerable time and money during 2008 and early 2009 attempting to raise money, but failed to conclude any transaction for reasons which remain unclear. The Company's inability to raise money put the company squarely in the position where it could only do a dilutive financing once its cash reserves had been depleted. This occurred in April.
The situation was compounded by longstanding inattention to overdue vendor payments -- including the Delaware Franchise Tax Board -- resulting in the Company's lack of good standing in Delaware.
-- Objective: As of April we estimated that it would require $2.0 million to operate the Company and complete the trial through the end of 2009, $480,000 to retire overdue payables to clinical trial sites, and $600,000 to retire debt. Since April, we have incurred unanticipated legal and other costs related to Len Brandt's actions which we estimate will exceed $300,000 by year end.
So, to run the company through year-end required a minimum of $3,380,000, leading us to conclude that an equity financing from $4-5 million would be necessary for the company to implement its plan. We have raised $1.2 million of that amount so far in convertible bridge notes, as outlined below.
-- Actions & Results:
- We secured a well-known, successful venture capitalist -- John
Pappajohn -- as lead investor. John brought our story and business
plans to institutions who will be critical to future equity
financing. Additionally, John invested $1 million in bridge loans
to the company, and SAIL Ventures invested another $200,000. These
funds were immediately employed to execute the operating objectives
described above.
- The company retired $150,000 in overdue vendor payables and $125,000
in company debt. The Company is fully paid up and in good standing
in Delaware.
- The practical effect of this effort has been to stabilize the
company, take 5 million shares off the capitalization table, and
position the company to raise equity at a better valuation than
would have been possible before.
"As much as we've done, we still have plenty of work to do. But we have a few advantages: we've unlocked the talents of a talented team, with more to come. And we've tapped the energies of our clinical 'power users,' our physicians, and we're listening to them.
"In this first 90 days, the major unplanned expense for the company has been the dispute with Len Brandt. In his attempt to force an immediate shareholder meeting to replace all directors and implement his strategy, he's distributed inaccurate and inconsistent information to current shareholders, and created potential violations of securities law. To defend the company from Len's actions, we estimate that we will incur over $300,000 in expenses by year-end.
"Curiously, all of the directors and employees that he seeks to remove are people that he himself brought in to the company, but who challenged his failure to properly capitalize and lead the company. Some shareholders I've spoken to were unaware that a Board change (as proposed by Brandt) would trigger an immediate repayment of $1.45 million in debt to SAIL and Pappajohn, in addition to the current operating and clinical trial costs through year-end of over $3.4 million (as detailed above). So unless Len Brandt has $5 million in hand at a significantly higher valuation than what has been considered by the Board, this dispute is an expensive waste of time and a risk to this incredible asset.
"Referenced-EEG® is a big, transformative idea. It's time that we give this idea the respect, capital, and professionalism that it will need to become a big, transformative product. So I'll continue to provide you regular updates like this, and welcome your thoughts on how to protect and grow our asset. I'm confident that if we continue to grow test volume, complete vital research like the Depression Efficacy trial, and build our database in the same constructive and relentless way we've approached our first 90 days, shareholder value will follow."
Sincerely, George Carpenter CEO
Additional Information and Where to Find It
This release may be deemed to be solicitation material in respect of the matters to be considered at the Company's 2009 Annual Meeting of Stockholders and/or the purported special meeting called by Mr. Brandt or his purported solicitation of written consents. The Company intends to file a proxy statement with the Securities and Exchange Commission ("SEC"). CNS SECURITYHOLDERS ARE URGED TO READ THE PROXY STATEMENT AND ANY OTHER RELEVANT DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. CNS Response Security holders will be able to receive the proxy statement and other relevant documents free of charge at the SEC's Web site at www.sec.gov or from the Company at 2755 Bristol Street, Suite 285, Costa Mesa, CA 92626.
Participants in Solicitation
CNS and its directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the matters to be considered at the Company's 2009 Annual Meeting of Stockholders and/or any purported special meeting called by Mr. Brandt or his purported solicitation of written consents. Information regarding the interests of the Company's directors and executive officers in the proxy contest will be included in its definitive proxy statement.
About CNS Response
Today, most physicians are able to base treatment on objective test data, such as EKGs, MRIs, blood tests, etc. Broadly speaking, such advances have not yet come to those physicians practicing psychiatry.
CNS Response has developed a patented data-analysis capability that, with the help of a simple, non-invasive EEG, will analyze a patient's brain waves and compare the results to an extensive patient outcomes database. The process produces a rEEG® report providing a psychiatrist with guidance to personalize medication regimens for a patient, based on the patient's own brain physiology. To read more about the benefits this patented technology provides physicians, patients and insurers, please visit the CNS Response website, www.cnsresponse.com.
Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995
Except for the historical information contained herein, the matters
discussed are forward-looking statements made pursuant to the safe harbor
provisions of the Private Securities Litigation Reform Act of 1995, as
amended. These statements involve risks and uncertainties as set forth in
the Company's filings with the Securities and Exchange Commission. These
risks and uncertainties could cause actual results to differ materially
from any forward-looking statements made herein.
Financial and Media Relations:
To be added to news release distribution, contact:
Marty Tullio
Managing Partner
McCloud Communications, LLC
949.553.9748
Email Contact