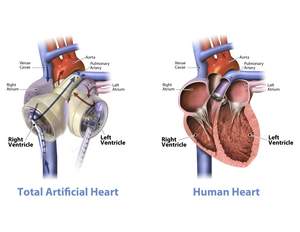
TUCSON, AZ--(Marketwire - March 25, 2011) - SynCardia Systems, Inc. announced today that the 13-member multi-disciplinary team from Cedars-Sinai Heart Institute will become the first in Los Angeles to complete certification to implant the SynCardia temporary Total Artificial Heart. During 2010, Cedars-Sinai performed the most heart transplants, 75, of all the 116 U.S. medical centers that performed adult heart transplants.
"We are excited to offer the Total Artificial Heart as a bridge to transplant for patients suffering from end-stage, biventricular heart failure who have exhausted all other treatment options," said Jaime Moriguchi, MD, medical director of the Mechanical Circulatory Support Program. "Similar to a heart transplant, the Total Artificial Heart is the only device that eliminates the symptoms and source of biventricular failure by replacing both ventricles and the four valves."
Said Fardad Esmailian, MD, surgical director for the Heart Transplant and Ventricular Assist Device Program, "Cedars-Sinai patients with advanced heart failure could begin receiving the Total Artificial Heart as early as next week."
SynCardia is currently conducting an FDA-approved Investigational Device Exemption (IDE) clinical study of the Freedom® driver, the first U.S. portable driver designed to power SynCardia's Total Artificial Heart both inside and outside the hospital. Weighing 13.5 lbs, the wearable Freedom driver is designed to allow stable Total Artificial Heart patients who meet study criteria to live at home and in their community while they wait for a matching donor heart.
U.S. News & World Report ranked Cedars-Sinai among the top 15 hospitals for Heart and Heart Surgery for 2010-2011. Since its establishment in 1988, the Heart Transplant Program at Cedars-Sinai has performed more than 650 heart transplants.
To become a SynCardia Certified Center, hospitals must apply for and complete a rigorous four-phase certification process, including the first implant proctored by an experienced Total Artificial Heart surgeon. Currently, there are 37 SynCardia Certified Centers around the world including 15 in the U.S. There are an additional 12 U.S. centers and 18 international hospitals currently completing the certification process.
About SynCardia Systems, Inc.
SynCardia Systems, Inc. is the Tucson-based manufacturer of the world's only FDA, Health Canada and CE approved Total Artificial Heart: the SynCardia temporary Total Artificial Heart. There have been more than 900 implants of the Total Artificial Heart, accounting for more than 210 patient years of life on the device.
Originally used as a permanent replacement heart, the Total Artificial Heart is currently approved as a bridge to human heart transplant for people dying from end-stage biventricular failure. The Total Artificial Heart is the only device that provides immediate, safe blood flow of up to 9.5 L/min through both ventricles.
For additional information, please visit: http://www.syncardia.com
or follow SynCardia on Twitter - @SynCardia_News
Media Contact:
Don Isaacs
Vice President of Communications
SynCardia Systems, Inc.
Cell: (520) 955-0660