Axon Therapies, a Coridea portfolio company focused on addressing one of the root causes of heart failure, today announced positive interim results from their first-in-human (FIH) trial of splanchnic ablation for volume management, or SAVM, using the Satera™ Ablation System.
|
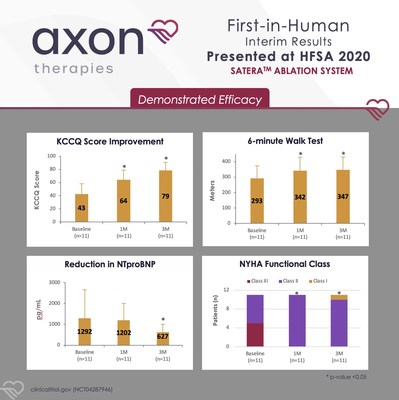
NEW YORK, Oct. 9, 2020 /PRNewswire/ -- Axon Therapies, a Coridea portfolio company focused on addressing one of the root causes of heart failure, today announced positive interim results from their first-in-human (FIH) trial of splanchnic ablation for volume management, or SAVM, using the Satera™ Ablation System. Dr. Sanjiv Shah, Director of the HFpEF program at Northwestern University Feinberg School of Medicine in Chicago, IL presented the results during the Late Breaking Clinical Trial session at the annual Heart Failure Society of America (HFSA) meeting. "As physicians, we are often limited in our ability to adequately decongest heart failure patients and prevent excessive increases in pressure during exertion. Although research has shown that inappropriate volume redistribution is a major contributor to functional limitations and outcomes, no interventions targeting this mechanism have existed until now," stated cardiologist Dr. Sanjiv Shah. "I'm very excited about the positive first-in-human results from Axon's innovative ablation technology. The SAVM procedure selectively blocks the neural pathway carrying signals from the chronically active sympathetic system to the splanchnic vascular bed, causing selective venodilation, improved venous compliance, and ultimately restoring appropriate volume balance within the circulation." A total of 11 heart failure patients with preserved ejection fraction (HFpEF) were successfully treated using Axon's Satera™ Ablation System. Interim data was presented on 1- and 3-month follow-up post-procedure. Endpoints included a composite safety index, New York Heart Association (NYHA) Heart Failure classification, Kansas City Cardiomyopathy Questionnaire (KCCQ) score, and 6-minute walk test. Study highlights include:
Building on the FIH results, Axon is initiating REBALANCE-HF, a prospective, randomized, sham controlled, double-blinded feasibility IDE trial in early 2021. The study will enroll 80 patients at up to 20 sites to assess the safety and efficacy of SAVM using the Satera Ablation System in HFpEF patients. Learn more at www.axontherapies.com. "The SAVM procedure is a frontline heart failure therapy that restores volume balance, stops disease progression and improves outcomes in heart failure patients," commented Howard Levin MD, CEO of Axon Therapies. "In addition, the outpatient, transvenous procedure is implant-free, which preserves future options for the patient. We are initially focusing on the underserved HFpEF patient population, but believe SAVM, with the Satera Ablation System, may have applications for all heart failure patients in the future." About Axon Therapies, Inc.
SOURCE Axon Therapies, Inc. |