SAN DIEGO, April 10, 2015 /PRNewswire/ -- Inova Diagnostics, the worldwide leader in autoimmune diagnostic reagents and systems for the clinical laboratory, announced today that the United States Food and Drug Administration (FDA) has cleared NOVA View, an automated digital IFA (indirect fluorescent assay) microscope, through the de novo classification process. NOVA View is the first FDA cleared automated digital IFA microscope in the USA, a long-awaited solution for laboratories that perform IFA for the detection of antinuclear antibodies (ANA). NOVA Lite® DAPI ANA Kit, an IFA reagent indicated for use with NOVA View, received 510(k) clearance at the same time.
ANA is the first line laboratory test for the diagnosis of systemic lupus erythematosus and other systemic autoimmune rheumatic diseases. Early and accurate diagnosis and effective treatment are key to reducing the morbidity associated with these conditions.1
IFA is the gold standard for ANA testing according to a Position Statement issued by the American College of Rheumatology in 2009.2 IFA, as it is currently practiced in many laboratories, is time and labor-intensive, subject to interpretation bias and other variables, such as the type of microscope and intensity of the light source. The workflow is manual, and prone to transcription errors.
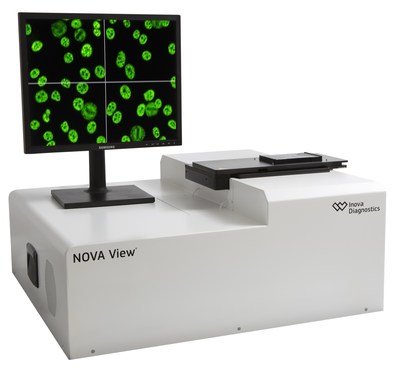
NOVA View addresses many shortcomings of the manual IFA process by reducing hands-on time, providing consistent reading and interpretation conditions, and generating digital images that can be archived. It provides patient sample traceability with positive patient identification and improves assay integrity by using NOVA Lite barcoded IFA slides.
"I am truly excited about the clearance of NOVA View," commented Marvin Fritzler, PhD, MD, Professor Cumming School of Medicine, University of Calgary and Director of Mitogen Advanced Diagnostics. "It is the fruition of a long anticipated technology breakthrough, marking a significant step forward in standardizing the ANA IFA test and providing clinicians with a reliable test result. In addition, the capability of providing an IFA test result that can be seamlessly uploaded into the patient's electronic medical record will be a remarkable advancement in prompt and accurate reporting."
"Inova Diagnostics is pleased to launch the first automated digital IFA microscope in the US," said Roger Ingles, Chief Executive Officer of Inova Diagnostics. "Inova Diagnostics has a 28 year history of providing laboratories with innovative products required for autoimmune diagnosis. NOVA View continues this tradition."
"NOVA View is a technology that fundamentally changes the way IFA is performed in diagnostic laboratories," said Gabriella Lakos, MD, PhD, Director of Assay Development. "NOVA View will bring efficiency and reliability to this traditionally labor intensive and subjective area of diagnostic immunology."
About NOVA View® and NOVA Lite® DAPI ANA Kit
NOVA View® Automated Fluorescence Microscope is an automated system consisting of a fluorescence microscope and software that acquires, analyzes, stores and displays digital images of stained indirect immunofluorescent slides. It is intended as an aid in the detection and classification of certain antibodies by indirect immunofluorescence technology. The device can only be used with cleared or approved in vitro diagnostic assays that are indicated for use with the device. A trained operator must confirm results generated with the device.
NOVA View determines the result (positive or negative), and performs ANA pattern interpretation. After pattern confirmation by the operator, NOVA View is able to predict a pattern specific endpoint titer. Results are recorded electronically in a transcription free and paperless work environment, and all digital images are archived for future review.
NOVA Lite® DAPI ANA Kit is an indirect immunofluorescence assay for the qualitative detection and semi-quantitative determination of anti-nuclear antibodies of the IgG isotype in human serum by manual fluorescence microscopy or with NOVA View Automated Fluorescence Microscope. The presence of anti-nuclear antibodies can be used in conjunction with other serological tests and clinical findings to aid in the diagnosis of systemic lupus erythematosus and other systemic rheumatic diseases. A trained operator must confirm results when generated with NOVA View device.
About Inova Diagnostics
Inova Diagnostics is a privately held company headquartered in San Diego, California, and is a part of Werfen, a global leader in in vitro diagnostics (IVD) with a long term commitment to providing high quality, innovative solutions for hospitals and clinical laboratories to enhance patient care. Inova Diagnostics manufactures IVD systems and reagents for autoimmune diseases that are used in clinical laboratories and hospitals around the world, and is a leader in the development and commercialization of new autoimmune technologies and diagnostic markers. Further information about Inova Diagnostics can be found at www.inovadx.com.
NOVA View and NOVA Lite are registered trademarks of Inova Diagnostics.
1. | Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis Published Online First: Oct 14, 2013 annrheumdis-2013-203863. |
2. | American College of Rheumatology, Position Statement, Methodology of Testing for Antinuclear Antibodies, 2009. |
Photo - http://photos.prnewswire.com/prnh/20150410/197847
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/nova-view-automated-digital-ifa-microscope-receives-fda-de-novo-clearance-300064306.html
SOURCE INOVA Diagnostics
 Help employers find you! Check out all the jobs and post your resume.
Help employers find you! Check out all the jobs and post your resume.




