HemoSonics Welcomes FDA Support of Viscoelastic Coagulation Analyzers for COVID-19 Patients; Highlights the Benefits of the Quantra ® Hemostasis Analyzer
HemoSonics Welcomes FDA Support of Viscoelastic Coagulation Analyzers for COVID-19 Patients; Highlights the Benefits of the Quantra® Hemostasis Analyzer
CHARLOTTESVILLE, Va.--(BUSINESS WIRE)-- HemoSonics welcomes the January 14th guidance of the US Food and Drug Administration (FDA) that broadens the use of viscoelastic coagulation analyzers for hospital patient healthcare settings in response to COVID-19. The FDA guidance is intended, for the duration of the COVID-19 public health emergency, to expand the availability of coagulation systems for measurement of whole blood viscoelastic properties to assess hemostasis.1
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210119005891/en/
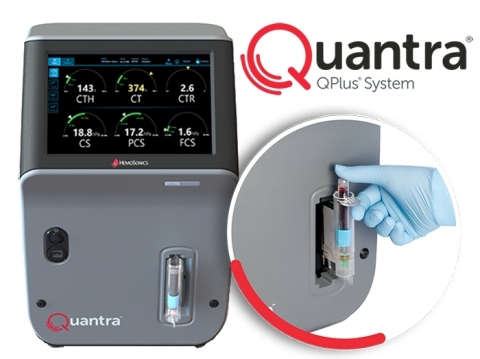
Quantra QPlus System: Quantra Hemostasis Analyzer, QPlus Cartridge. (Photo: Business Wire)
HemoSonics’s flagship product, the Quantra Hemostasis Analyzer, with its QPlus® cartridge, represents the only viscoelastic coagulation testing (VET) system specifically designed and optimized for rapid and easy operation in point-of-care (POC) settings, such as operating rooms, Emergency Departments, or intensive care units. The Quantra system’s innovative closed-cartridge design requires no open-tube blood manipulation after sample collection. The Quantra system is FDA cleared (de novo) for POC use, which may help to minimize the flow of traffic with sample transporters and/or operators in and out of units designated for the care of COVID-19 patients.
“The Quantra System’s intuitive operation and easy-to-interpret Dials display may help streamline what is otherwise a complex process of coagulation testing,” said Bob Roda, CEO of HemoSonics. “The QPlus cartridge provides multiple whole blood coagulation parameters within 15 minutes or less, helping clinicians to make quicker, more informed treatment decisions that support better patient outcomes.”
Hypercoagulability, an abnormally increased risk for blood clotting, has been observed in patients with COVID-19.2 Clinical reports have shown that in patients with severe COVID-19 infection, blood clotting abnormalities are likely triggered by the acute inflammatory response.3-7
HemoSonics has advocated for the use of viscoelastic whole blood testing as a tool in the diagnosis and management of COVID-19 patients, consistent with its mission to provide efficient, easy-to-use tools that improve patient care.
Since the onset of the global pandemic, HemoSonics has supported several clinical sites in Europe and the US in their quest to understand the potential utility of the Quantra system for the evaluation of coagulation status in COVID-19 patients at the POC setting:7
“The Quantra is the only device on which we can operate safely without needing specialized rooms and a biohazard cabinet.”
– Ekaterina Baryshnikova, PhD, IRCCS Policlinico San Donato, Milan, Italy
The Quantra system’s innovative ultrasound technology, proprietary closed-cartridge design, unparalleled ease of use, and ease of interpretation make it uniquely suited for use in critical or emergency care settings with COVID-19 patients.
For more information on the Quantra QPlus System, email contact@hemosonics.com or visit www.HemoSonics.com.
Disclaimer
This communication contains information on products which is targeted to a wide range of audiences and could contain product details or information not otherwise accessible or valid in your country. Please refer to the applicable approved instructions for use.
About HemoSonics
HemoSonics is a medical device company with the primary mission to deliver clinical tools that provide actionable information in the critical care settings, resulting in better care for patients and lower overall medical costs. The Quantra® Hemostasis Analyzer, the flagship product of HemoSonics, is designed to improve patient outcomes and reduce healthcare costs by providing unique and optimized coagulation information, for easy and fast interpretation and simple, more efficient point-of-care bleeding management workflows. HemoSonics is headquartered in Charlottesville, VA, with research, development and manufacturing facilities in Durham, NC. HemoSonics is part of the Stago group, a leading company in the in vitro diagnostics industry dedicated to the exploration of thrombosis and hemostasis.
References:
1. US Food and Drug Administration. Coagulation Systems for Measurement of Viscoelastic Properties: Enforcement Policy During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency: Guidance for Industry and Food and Drug Administration Staff, January 2021. Available at: https://www.fda.gov/media/145135/download.
2. Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
3. Han H, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020 Jun 25;58(7):1116-1120. doi: 10.1515/cclm-2020-0188.
4. Tang N, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-47.
5. Darzi AJ, et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: a systematic review and meta-analysis. Blood. 2020 May 14;135(20):1788-1810. doi: 10.1182/blood.2019003603.
6. Hunt B, et al. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. https://thrombosisuk.org/covid-19-thrombosis.php.
7. Ranucci M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 Jul;18(7):1747-1751. doi: 10.1111/jth.14854.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210119005891/en/
Contacts
Janna Badalian
Manager, Marketing Communications
jbadalian@hemosonics.com
+1 (919) 386-3186
Source: HemoSonics