Gossamer Bio Announces Presentation of GB004 Phase 1b Clinical Data at United European Gastroenterology Virtual Week 2020 and Additional Program Updates
- Newly disclosed data build on topline results and continue to support GB004’s differentiated approach of targeting epithelial barrier restoration in patients with ulcerative colitis -
- GB004 demonstrated superior barrier protection compared to tofacitinib in pre-clinical human monolayer assay -
- SHIFT-UC Phase 2 study active and screening patients for enrollment -
SAN DIEGO--(BUSINESS WIRE)-- Gossamer Bio. (Nasdaq: GOSS), today announced multiple updates for its GB004 program. GB004 is an oral, gut-targeted HIF-1α stabilizer, designed to promote mucosal healing and resolve local inflammation through a non-immunosuppressive mechanism of action in patients with inflammatory bowel disease.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201012005210/en/
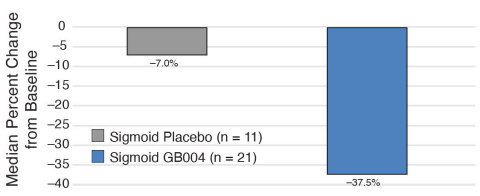
Median Reduction from Baseline in Fecal Calprotectin (Graphic: Business Wire)
Data Presented at UEGW Virtual 2020
William Sandborn, M.D., Chief of the Division of Gastroenterology of University of California San Diego, presented data at the UEGW Virtual Week 2020 from the successful Phase 1b translational medicine study of GB004 in mild-to-moderate ulcerative colitis, or UC.
The Phase 1b study evaluated safety, tolerability, and pharmacokinetics over 4 weeks of treatment in patients with active UC despite treatment with 5-aminosalicylate (5-ASA) therapy. In addition to trends disclosed earlier this year, the presentation included newly disclosed data on the reduction of an important inflammatory biomarker in IBD, fecal calprotectin, and the resolution of rectal bleeding, which is considered to be an objective measure of disease activity.
|
Key Exploratory Outcomes |
||
|
Outcome / Biomarker (4 weeks) |
Placebo |
GB004 |
|
Mucosal Healing |
0% (0/11) |
17% (4/23) |
|
Histologic Remission |
18% (2/11) |
43% (10/23) |
|
Resolution of Rectal Bleeding |
36% (4/11) |
57% (12/21) |
|
Reduction from Baseline in Fecal Calprotectin |
7% (n=11) |
38% (n=21) |
GB004’s differentiated mechanism of action was also highlighted in a second poster summarizing data from two pre-clinical studies. In a human monolayer assay, GB004 demonstrated superior protection of barrier integrity compared to tofacitinib, a pan-JAK inhibitor approved for the treatment of moderate-to-severe UC. In a separate mouse organoid study, GB004 induced HIF-1α-dependent genes, including barrier function genes such as Claudin 1, which were elevated in patients in the Phase 1b trial, further validating GB004’s mechanism of action.
Both posters presented at UEGW Virtual Week 2020 and a replay of Dr. Sandborn’s presentation can now be viewed in the “Posters & Publications” of Gossamer’s website at https://www.gossamerbio.com/pipeline/posters-and-publications/.
GB004 SHIFT-UC Phase 2 Study in Patients with Active Mild-to-Moderate UC
Gossamer has begun screening patients in a global Phase 2 study of GB004 in patients with active ulcerative colitis despite treatment with 5-ASA (NCT04556383). Up to 195 subjects are expected to be enrolled, randomized evenly across two dose arms of GB004 and placebo.
The primary endpoint of the study is clinical remission at week 12. Patients will continue blinded treatment through week 36, after which they will have the option to roll onto an open-label extension. Additional endpoints such as mucosal healing, histologic remission, clinical response, and disease clearance also will be assessed.
About Gossamer Bio
Gossamer Bio is a clinical-stage biopharmaceutical company focused on discovering, acquiring, developing and commercializing therapeutics in the disease areas of immunology, inflammation and oncology. Its goal is to be an industry leader in each of these therapeutic areas and to enhance and extend the lives of patients suffering from such diseases.
Forward-Looking Statements
Gossamer cautions you that statements contained in this press release regarding matters that are not historical facts are forward-looking statements. These statements are based on the Company’s current beliefs and expectations. Such forward-looking statements include, but are not limited to, statements regarding plans to advance our product candidates and the potential clinical benefits of our product candidates. The inclusion of forward-looking statements should not be regarded as a representation by Gossamer that any of its plans will be achieved. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in Gossamer’s business, including, without limitation: potential delays in the commencement, enrollment and completion of clinical trials; disruption to our operations from the recent global outbreak of the COVID-19 pandemic, including clinical trial delays; the Company’s dependence on third parties in connection with product manufacturing, research and preclinical and clinical testing; the results of preclinical studies and early clinical trials are not necessarily predictive of future results; the success of Gossamer’s clinical trials and preclinical studies for its product candidates; interim results do not necessarily predict final results and one or more of the outcomes may materially change as the trial continues and more patient data become available and following more comprehensive audit and verification procedures; regulatory developments in the United States and foreign countries; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval and/or commercialization, or may result in recalls or product liability claims; Gossamer’s ability to obtain and maintain intellectual property protection for its product candidates; Gossamer’s ability to comply with its obligations in collaboration agreements with third parties or the agreements under which it licenses intellectual property rights from third parties; Gossamer may use its capital resources sooner than it expects; and other risks described in the Company’s prior press releases and the Company’s filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in the Company’s annual report on Form 10-K and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Gossamer undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201012005210/en/
Contacts
For Investors and Media:
Bryan Giraudo, Chief Financial Officer
Gossamer Bio Investor Relations
ir@gossamerbio.com
Source: Gossamer Bio, Inc.