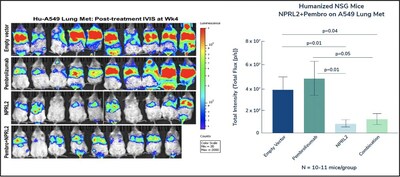
Genprex, Inc. today announced that its research collaborators have published positive preclinical data for the NPRL2 tumor suppressor gene, utilizing the Company’s non-viral Oncoprex® Delivery System, in KRAS/STK11 mutant anti-PD1 resistant non-small cell lung cancer (NSCLC) in a humanized mouse model.
|
NPRL2 Gene Therapy Induces Anti-Tumor Activity in Anti-PD1 Resistant KRAS/STK11 Mutant Non-Small Cell Lung Cancer in a Humanized Mouse Model Provides Additional Preclinical Validation of Oncoprex® Delivery System With Another Tumor Suppressor Gene AUSTIN, Texas, April 2, 2024 /PRNewswire/ --Genprex, Inc. ("Genprex" or the "Company") (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that its research collaborators have published positive preclinical data for the NPRL2 tumor suppressor gene, utilizing the Company's non-viral Oncoprex® Delivery System, in KRAS/STK11 mutant anti-PD1 resistant non-small cell lung cancer (NSCLC) in a humanized mouse model. NPRL2 is a tumor suppressor gene whose expression is reduced in many cancers including lung, renal, colorectal, glioma, gastric, and hepatocellular carcinoma, and it has been closely correlated with poor clinical outcomes. Genprex's Oncoprex® Delivery System is a novel non-viral approach that utilizes lipid-based nanoparticles in a lipoplex form to deliver tumor suppressor genes deleted during the course of cancer development. The platform allows for the intravenous delivery of various tumor suppressor genes, and potentially other genes, to achieve a therapeutic affect without the risk of toxicity often associated with viral delivery systems. Genprex believes this system allows for delivery of a number of cancer-fighting genes, alone or in combination with other cancer therapies, to combat multiple types of cancer. The manuscript, titled, "NPRL2 gene therapy induces effective anti-tumor immunity in KRAS/STK11 mutant anti-PD1 resistant metastatic non-small cell lung cancer (NSCLC) in a humanized mouse model," was published on the bioRxiv biology preprint server. "These positive preclinical data are very encouraging and support NPRL2 gene therapy as a potential treatment for a sub-group of NSCLC in which patients traditionally are resistant to existing therapies," said Rodney Varner, President, Chairman and Chief Executive Officer at Genprex. "We believe this data could support the potential for a new drug candidate in our pipeline, and it also provides further evidence that the Oncoprex® Delivery System has the ability to be successful using genes other than the TUSC2 gene we are already using in clinical trials with Reqorsa®." The studies evaluated the intravenous injection of NPRL2 gene-loaded cationic lipoplexes (DOTAP-NPRL2) with or without anti-PD1 drugs (pembrolizumab). The studies used a KRAS/STK11 mutant anti-PD1 insensitive cell line, as well as syngeneic mouse LLC2 tumors, which are also anti-PD1 resistant. In both of these mouse models, NPRL2 showed a significantly strong anti-tumor effect whereas anti-PD1 (pembrolizumab) was not effective. The anti-tumor effect was greater in humanized mice than non-humanized mice, suggesting that an immune response contributed to anti-tumor activity. Additionally, a dramatic anti-tumor effect was mediated by NPRL2 treatment with or without a pembrolizumab combination. Bioluminescence imaging on mice showed that 7 out of 10 mice contained an extremely low amount of tumor burden in the NPRL2 treatment group, which was significantly different than in the control or pembrolizumab group. Unlike previous experiments with Reqorsa® Immunogene Therapy (quartusugene ozeplasmid), the Company's lead drug candidate using the TUSC2 tumor suppressor gene, the anti-tumor efficacy of DOTAP-NPRL2 did not involve Natural Killer (NK) cells. The studies also found that tumors with stable NPRL2 expression exhibited significantly slower growth compared to controls. In conclusion, researchers reported that NPRL2 gene therapy induces anti-tumor activity through dendritic cell-mediated antigen presentation and cytotoxic immune cell activation. About Genprex, Inc. Interested investors and shareholders are encouraged to sign up for press releases and industry updates by visiting the Company Website, registering for Email Alerts and by following Genprex on Twitter, Facebook and LinkedIn. Cautionary Language Concerning Forward-Looking Statements Because forward-looking statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: Genprex's ability to advance the clinical development, manufacturing and commercialization of its product candidates in accordance with projected timelines and specifications; the timing and success of Genprex's clinical trials and regulatory approvals; the effect of Genprex's product candidates, alone and in combination with other therapies, on cancer and diabetes; Genprex's future growth and financial status, including Genprex's ability to maintain compliance with the continued listing requirements of The Nasdaq Capital Market and to continue as a going concern and to obtain capital to meet its long-term liquidity needs on acceptable terms, or at all; Genprex's commercial and strategic partnerships, including those with its third party vendors, suppliers and manufacturers and their ability to successfully perform and scale up the manufacture of its product candidates; and Genprex's intellectual property and licenses. These forward-looking statements should not be relied upon as predictions of future events and Genprex cannot assure you that the events or circumstances discussed or reflected in these statements will be achieved or will occur. If such forward-looking statements prove to be inaccurate, the inaccuracy may be material. You should not regard these statements as a representation or warranty by Genprex or any other person that Genprex will achieve its objectives and plans in any specified timeframe, or at all. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. Genprex disclaims any obligation to publicly update or release any revisions to these forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release or to reflect the occurrence of unanticipated events, except as required by law. Genprex, Inc. GNPX Investor Relations GNPX Media Contact
SOURCE Genprex, Inc. |
||
Company Codes: NASDAQ-NMS:GNPX |