4C Medical Technologies, Inc., the creator of a new generation of therapies for structural heart disease, announced that its medical device therapy for mitral regurgitation, AltaValve™, was featured at Transcatheter Cardiovascular Therapeutics 2019, on Sept 25-29 in San Francisco, CA.
MAPLE GROVE, Minn., Sept. 30, 2019 /PRNewswire/ -- 4C Medical Technologies, Inc., the creator of a new generation of therapies for structural heart disease, announced today that its medical device therapy for mitral regurgitation (MR), AltaValve™, was featured at Transcatheter Cardiovascular Therapeutics (TCT) 2019, on Sept 25-29 in San Francisco, CA.
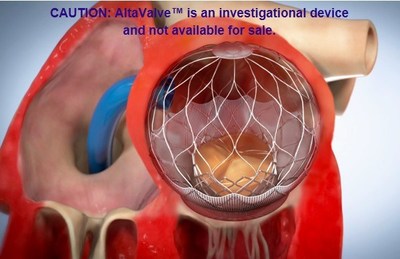
The AltaValve is a Transcatheter Mitral Valve Replacement (TMVR) device designed to broaden the treatable patient population: the device's supra-annular fit and atrial-only fixation bypass the concerns of anchoring and fixation difficulties present in current TMVR technologies. Moreover, AltaValve leaves the left ventricle geometry intact and minimizes the risks of left ventricular outflow tract (LVOT) obstruction and damage to the left ventricle.
"AltaValve is the only device that truly differentiated itself in a crowded TMVR market. Delivered transseptally, AltaValve can be implanted using a very simple procedure, suitable for the vast majority of patients suffering of MR. Due to its unique supra-annular design, more patients will qualify for treatment with AltaValve. More importantly, AltaValve has the potential to be the only TMVR that could be implanted in patients with previously failed mitral edge-to-edge repair," said Philippe Généreux, MD, Co-Director of the Structural Heart Program at the Gagnon Cardiovascular Institute of Morristown Medical Center (Morristown, NJ). "AltaValve is truly an innovative, scalable technology that answers a real unmet need. It has the potential to become the TAVR of the mitral space."
Dr. Généreux highlighted AltaValve in the presentation titled "AltaValve - Device Description, Results, and Ongoing Studies" which was part of the TMVR Overview and Principle Devices session.
"It's no easy task to redefine solutions for structural heart disease, but we're doing it. We've continued our commitment to improve quality of life for patients with mitral regurgitation (MR)," said Robert Thatcher, CEO of 4C Medical Technologies, Inc.
About 4C Medical Technologies, Inc.
4C Medical Technologies, Inc. is a medical device company developing innovative therapies for structural heart disease, focusing initially on MR therapy and subsequently on tricuspid regurgitation therapy. The company's unique supra-annular implant is the first MR therapy with atrial-only fixation, thereby minimizing known issues associated with current TMVR technologies which rely on placement and fixation in the native mitral annulus and left ventricle.
- Nkomo VT, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-11. doi: 10.1016/S0140-6736(06)69208-8.
Media Contacts
Robert Thatcher
Chief Executive Officer
(612) 600-8951
rthatcher@4CMed.com
Jim Flaherty
Chief Financial Officer
(612) 599-0650
jflaherty@4CMed.com

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/altavalve-4c-medicals-novel-transcatheter-mitral-regurgitation-device-highlighted-at-tct-2019-300927416.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/altavalve-4c-medicals-novel-transcatheter-mitral-regurgitation-device-highlighted-at-tct-2019-300927416.html
SOURCE 4C Medical Technologies, Inc.




