BRIDGEWATER, N.J., June 14, 2016 /PRNewswire/ -- Ajanta Pharma USA Inc., a wholly owned subsidiary of Ajanta Pharma Ltd, announces today the launch of Memantine Hydrochloride Tablets (5mg, 10mg), a bioequivalent generic version of Namenda®, into the US market.
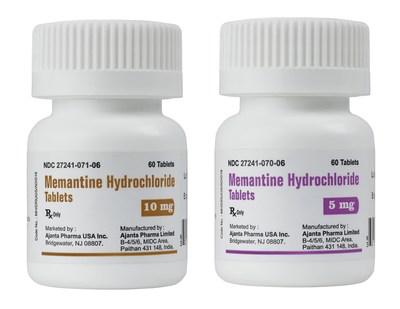
Memantine Hydrochloride Tablets is part of an ever-growing portfolio of products that Ajanta has developed for the US market. To date, the United States Food & Drug Administration (FDA) has granted Ajanta Pharma 10 final Abbreviated New Drug Application (ANDA) approvals and 2 tentative approvals. An additional 14 ANDAs are pending approval from the FDA.
About Ajanta Pharma
Ajanta Pharma Limited is a fully-integrated pharmaceutical company with global headquarters in Mumbai, India. Over 6,000 employees are engaged in developing, manufacturing and marketing quality, pharmaceuticals across 35 countries. Its mission is to serve global healthcare needs through empathy, innovation and technology.
For more details about Ajanta Pharma USA Inc., please visit us at www.ajantapharmausa.com.
Namenda® is a registered trademark of Forest Laboratories, Inc.
Photo - http://photos.prnewswire.com/prnh/20160614/378950
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/ajanta-pharma-announces-the-launch-of-memantine-hydrochloride-tablets-300284337.html
SOURCE Ajanta Pharma USA Inc.





