Hamsters Receiving Ainos’ VELDONA Formulation Demonstrate Protection from COVID-19 Based on Prespecified Indicators of Clinical Outcomes.
|
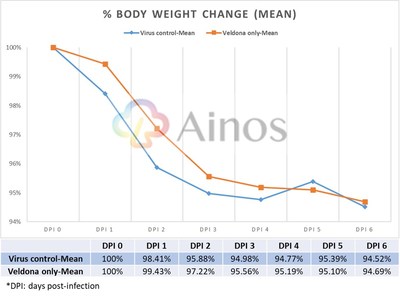
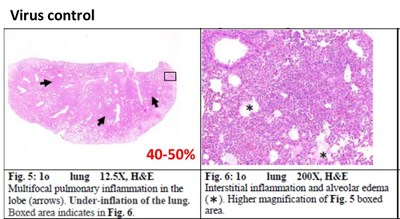
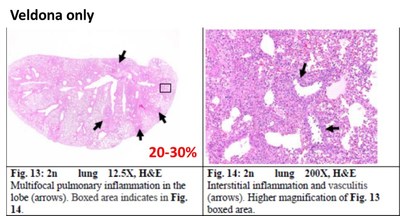
Hamsters Receiving Ainos' VELDONA Formulation Demonstrate Protection from COVID-19 Based on Prespecified Indicators of Clinical Outcomes SAN DIEGO, June 27, 2022 /PRNewswire/ -- Ainos, Inc. (OTC PINK: AIMD) ("Ainos", or the "Company"), a diversified medtech company focused on the development of novel point-of-care testing, low-dose interferon therapeutics, and synthetic RNA-driven preventative medicine, today announced the topline results from its COVID-19 antiviral efficacy study in hamsters (the "Study"). The available results show that its low-dose oral interferon alpha ("IFN-α") formulation, VELDONA, has a protective effect on lungs infected with the SARS-CoV-2 (the "Delta variant") virus by regulating the immune response, thus expediting recovery of infected animals. The Company will begin to prepare the application documents for U.S. FDA Phase 2/3 clinical trials. Further information regarding the Study can be found on the Company's website (https://www.ainos.com/support/en/listbypid/18). The Study evaluates the effectiveness of VELDONA over a seven-day course of treatment of Delta-variant-infected hamsters. Compared with hamsters in the control group receiving solution without VELDONA (the "Control Group"), the hamsters in the group receiving solution with VELDONA (the "VELDONA Group") demonstrated resistance to body weight loss during the first four days, then showed a similar recovery trend in the following two days. The body weights of the hamsters in the VELDONA Group remained more stable than those of the hamsters in the Control Group. Among the symptoms exhibited by hamsters infected with SARS-CoV-2 are interferon dysregulation and loss of body weight. VELDONA increases the secretion of interferon in the body, thereby enhancing immunoreaction. Percentage of Weight Change Delta variant viral infection mainly occurs in the upper respiratory tract, but in severe cases it will extend to the lower respiratory tract (i.e., the lungs) causing serious irreversible complications. The Study found that on the sixth day after infection, the amount of virus in the nasal cavities of the hamsters in the VELDONA Group declined significantly, with a maximum reduction ratio of 551.16. More importantly, hamsters in the VELDONA Group had less virus present in the lungs on the third day, and on the sixth day, the hamsters in the VELDONA Group demonstrated a significant reduction in virus, with a maximum reduction ratio of 720510.66. For pathological indicators, on the sixth day, the hamsters in the VELDONA Group showed 20-30% mixed-cellular inflammation, peribronchial infiltration, and perivascular infiltration, compared to 40-50% in the Control Group. Hamsters in the VELDONA Group in general showed promising results in treating indicators of mild/moderate COVID-19 infection, indicating viral clearance in moderate severity pneumonia (40-50%). The viral qPCR showed viral load (0-6175) in the VELDONA Group compared to viral load (7-21258) in the Control Group. Lung Pathology Report -Mixed cellular inflammation, peribronchial infiltration, and perivascular infiltration
"A pre-clinical animal study we conducted since March of this year validated the effectiveness of our low-dose oral interferon to protect against COVID-19. We replicated this study in May, with double the sample size, and obtained the same confirmative outcomes. These consistent results support the efficacy potential in inducing significant systemic immunomodulatory to fight against the COVID-19 virus. We believe that VELDONA may have the potential to become an important tool and a viable solution for the treatment of COVID-19 and other viral infections in the future. In addition, we also want to thank both the Taiwan Ministry of Science and Technology and the Emerging Infectious Disease Core Facility Platform of the National Core Facility for Biopharmaceuticals (NCFB) for their support and professional technical services provided in the Study." said Chun-Hsien Tsai, Ainos' Chairman of the Board, President, and Chief Executive Officer. About Ainos, Inc. Headquartered in San Diego, California, Ainos, Inc. (f/k/a Amarillo Biosciences, Inc.) is a diversified medtech company engaged in developing innovative medical technologies for point-of-care testing and safe and novel medical treatment for a broad range of disease indications. In addition to its proprietary therapeutics using low-dose non-injectable interferon, Ainos is committed to developing a comprehensive healthcare business portfolio encompassing medical devices and consumer healthcare products. While prioritizing the commercialization of medical devices as part of its diversification strategy, Ainos has also expanded its product portfolio to include Volatile Organic Compounds (VOC) and COVID-19 POCTs. Leveraging its patents related to VOC technologies and COVID-19 POCT products, the Company seeks to expedite the commercialization of its medical device pipeline, beginning with Ainos-branded COVID-19 POCT product candidates. Forward-Looking Statements This press release contains "forward-looking statements" about Ainos within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by the use of words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict," "project," "target," "future," "likely," "strategy," "foresee," "may," "guidance," "potential," "outlook," "forecast," "should," "will" or other similar words or phrases. Similarly, statements that describe the Company's objectives, plans or goals are, or may be, forward-looking statements. Forward-looking statements are based only on the Company's current beliefs, expectations, and assumptions. Forward-looking statements are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of the Company's control. The Company's actual results may differ materially from those indicated in the forward-looking statements. Important factors that could cause the Company's actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release include, among others, the cost of production and sales potential of the planned drug treatments announced in this press release; the Company's dependence on revenues from the sale of COVID-19 test kits; the Company's limited cash and history of losses; the Company's ability to achieve profitability; the Company's ability to raise additional capital to continue the Company's product development; the ability to accurately predict the future operating results of the Company; the ability to advance Ainos' current or future product candidates through clinical trials, obtain marketing approval and ultimately commercialize any product candidates the Company develops; the ability to obtain and maintain regulatory approval of Ainos product candidates; delays in completing the development and commercialization of the Company's current and future product candidates, which could result in increased costs to the Company, delay or limit the ability to generate revenue and adversely affect the business, financial condition, results of operations and prospects of the Company; intense competition and rapidly advancing technology in the Company's industry that may outpace its technology; customer demand for the products and services the Company develops; the impact of competitive or alternative products, technologies and pricing; disruption in research and development facilities; lawsuits and other claims by third parties or investigations by various regulatory agencies governing the Company's operations; potential cybersecurity attacks; increased requirements and costs related to cybersecurity; the Company's ability to realize the benefits of third party licensing agreements; the Company's ability to obtain and maintain intellectual property protection for Ainos product candidates; compliance with applicable laws, regulations and tariffs; and the Company's success in managing the growth. A more complete description of these risk factors and others is included in the "Risk Factors" section of Ainos' most recent Annual Report on Form 10-K/A and other reports filed with the U.S. Securities and Exchange Commission, many of which risks are beyond the Company's control. In addition to the risks described above and in the Company's Form 10-K/A, other unknown or unpredictable factors also could cause actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release. The forward-looking statements made in this press release are expressly qualified in their entirety by the foregoing cautionary statements. Ainos undertakes no obligation to, and expressly disclaims any such obligation to, publicly update or revise any forward-looking statement to reflect changed assumptions, the occurrence of anticipated or unanticipated events or changes to the future results over time or otherwise, except as required by law. Investor Relations Contact ICR, LLC
SOURCE Ainos, Inc. |
||
Company Codes: OTC-BB:AIMD, OTC-PINK:AIMD |