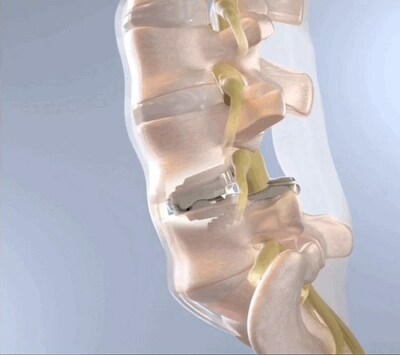
Pinehurst Surgical Clinic today announced successful completion of the first series of cases in the BalancedBack Total Joint Replacement pivotal clinical trial using the breakthrough 3Spine MOTUS device, an alternative to spinal fusion surgery.
|
PINEHURST, N.C., March 6, 2023 /PRNewswire/ -- Pinehurst Surgical Clinic today announced successful completion of the first series of cases in the BalancedBack Total Joint Replacement pivotal clinical trial using the breakthrough 3Spine MOTUS device, an alternative to spinal fusion surgery. The surgeries were performed at FirstHealth Moore Regional Hospital. Pinehurst Surgical Clinic is the first site in North Carolina to complete total joint replacement surgeries for the spine. 3Spine's lumbar total joint replacement procedure and MOTUS device is a "first of its kind" technology, replacing the function of the disc and facet joints through a posterior approach. The procedure is intended to broadly address leg pain, back pain and spinal instability while correcting posture and movement. Much like a hip or knee total joint replacement, the procedure treats degenerative disease through reconstruction of the functional spinal unit. Alexander C. Lemons, M.D., performed the first case together with his partner Daniel Williams, M.D. Dr. Lemons said, "I am thrilled to participate in this monumental clinical trial. MOTUS lumbar total joint replacement has the potential to significantly enhance quality of life for patients who suffer from leg and back pain. I believe this is the motion alternative to spinal fusion we have needed in our industry for decades." Charles Gregg, CEO of Pinehurst Surgical Clinic, said, "Our clinic was founded on true dedication to quality patient care. As part of that mission, we are honored to participate in a clinical trial that has the potential to help enhance the standard of care for patients dealing with leg and back pain. I congratulate Dr. Lemons, Dr. Williams, and the surgical team on their achievement." 3Spine, Inc., the study sponsor, is seeking single-level indications from L1-S1 in patients suffering from lumbar degeneration with or without foraminal or recess spinal stenosis with no more than a grade 1 spondylolisthesis at the involved level. Additional details are available at ClinicalTrials.gov. Patients interested in participating in the clinical trial in Pinehurst, North Carolina can email celliott@pinhurstsurgical.com for more information. About 3Spine: 3Spine is a new kind of healthcare company founded to integrate the development, clinical research, and delivery of low back total joint replacement. 3Spine is headquartered in Chattanooga, Tennessee, with research and development facilities in the Greater Boston area. About Pinehurst Surgical Clinic: Pinehurst Surgical Clinic, in Pinehurst, North Carolina, has been providing quality healthcare for more than 75 years and has grown to an active, board-certified physician staff of 56, with 30 mid-level providers and a professional staff of over 550, offering specialty services in 11 departments. In addition to the main clinic in Pinehurst, the professional healthcare staff services a five-county primary service area and a ten-county secondary service area. They offer specialty services at eight location areas in the counties of Moore, Scotland, Lee, Hoke, Montgomery, Troy, Cumberland, and Richmond. https://pinehurstsurgical.com/
SOURCE 3Spine |